Luminescent sensing of conformational integrin activation in living cells
- PMID: 39964812
- PMCID: PMC11861568
- DOI: 10.1016/j.celrep.2025.115319
Luminescent sensing of conformational integrin activation in living cells
Abstract
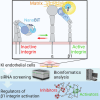
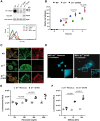
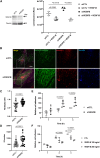
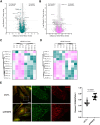
Integrins are major receptors for secreted extracellular matrix, playing crucial roles in physiological and pathological contexts, such as angiogenesis and cancer. Regulation of the transition between inactive and active conformation is key for integrins to fulfill their functions, and pharmacological control of those dynamics may have therapeutic applications. We create and validate a prototypic luminescent β1 integrin activation sensor (β1IAS) by introducing a split luciferase into an activation reporting site between the βI and the hybrid domains. As a recombinant protein in both solution and living cells, β1IAS accurately reports β1 integrin activation in response to (bio)chemical and physical stimuli. A short interfering RNA (siRNA) high-throughput screening on live β1IAS knockin endothelial cells unveils hitherto unknown regulators of β1 integrin activation, such as β1 integrin inhibitors E3 ligase Pja2 and vascular endothelial growth factor B (VEGF-B). This split-luciferase-based strategy provides an in situ label-free measurement of integrin activation and may be applicable to other β integrins and receptors.
Keywords: CP: Cell biology; angiogenesis; conformational sensor; endothelial cells; integrin activation; integrin inhibitors; luminescence.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

