Tumor-immune partitioning and clustering algorithm for identifying tumor-immune cell spatial interaction signatures within the tumor microenvironment
- PMID: 39965007
- PMCID: PMC11849983
- DOI: 10.1371/journal.pcbi.1012707
Tumor-immune partitioning and clustering algorithm for identifying tumor-immune cell spatial interaction signatures within the tumor microenvironment
Abstract
Background: Growing evidence supports the importance of characterizing the organizational patterns of various cellular constituents in the tumor microenvironment in precision oncology. Most existing data on immune cell infiltrates in tumors, which are based on immune cell counts or nearest neighbor-type analyses, have failed to fully capture the cellular organization and heterogeneity.
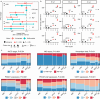
Methods: We introduce a computational algorithm, termed Tumor-Immune Partitioning and Clustering (TIPC), that jointly measures immune cell partitioning between tumor epithelial and stromal areas and immune cell clustering versus dispersion. As proof-of-principle, we applied TIPC to a prospective cohort incident tumor biobank containing 931 colorectal carcinoma cases. TIPC identified tumor subtypes with unique spatial patterns between tumor cells and T lymphocytes linked to certain molecular pathologic and prognostic features. T lymphocyte identification and phenotyping were achieved using multiplexed (multispectral) immunofluorescence. In a separate hepatocellular carcinoma cohort, we replaced the stromal component with specific immune cell types-CXCR3+CD68+ or CD8+-to profile their spatial relationships with CXCL9+CD68+ cells.
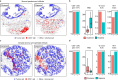
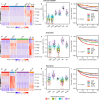
Results: Six unsupervised TIPC subtypes based on T lymphocyte distribution patterns were identified, comprising two cold and four hot subtypes. Three of the four hot subtypes were associated with significantly longer colorectal cancer (CRC)-specific survival compared to a reference cold subtype. Our analysis showed that variations in T-cell densities among the TIPC subtypes did not strictly correlate with prognostic benefits, underscoring the prognostic significance of immune cell spatial patterns. Additionally, TIPC revealed two spatially distinct and cell density-specific subtypes among microsatellite instability-high colorectal cancers, indicating its potential to upgrade tumor subtyping. TIPC was also applied to additional immune cell types, eosinophils and neutrophils, identified using morphology and supervised machine learning; here two tumor subtypes with similarly low densities, namely 'cold, tumor-rich' and 'cold, stroma-rich', exhibited differential prognostic associations. Lastly, we validated our methods and results using The Cancer Genome Atlas colon and rectal adenocarcinoma data (n = 570). Moreover, applying TIPC to hepatocellular carcinoma cases (n = 27) highlighted critical cell interactions like CXCL9-CXCR3 and CXCL9-CD8.
Conclusions: Unsupervised discoveries of microgeometric tissue organizational patterns and novel tumor subtypes using the TIPC algorithm can deepen our understanding of the tumor immune microenvironment and likely inform precision cancer immunotherapy.
Copyright: © 2025 Lau et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
We have read the journal’s policy and the authors of this manuscript have the following competing interests: C.S.F. is currently employed by Genentech, a subsidiary of Roche, and previously served as a consultant for Agios, Bain Capital, Bayer, Celgene, Dicerna, Five Prime Therapeutics, Gilead Sciences, Eli Lilly, Entrinsic Health, Genentech, KEW, Merck, Merrimack Pharmaceuticals, Pfizer Inc, Sanofi, Taiho, and Unum Therapeutics. J.A.M. has also served as an advisor/consultant to Ignyta, Array Pharmaceutical, and Cota. J.A.N. receives research funding from Natera and serves as a consultant for Leica Biosystems. J.K.L. is currently employed by BostonGene and owns company’s stock options. D.T. reports receiving research support from Novartis, Sirtex, and Bristol Myers Squibb. D.T. reports having received honorarium as an advisor for Novartis, Celgene, Sirtex, Merck Sharp & Dohme, Eisai, Ipsen, Bayer, and Bristol Myers Squibb.
Figures





References
-
- Abboud K, Umoru G, Esmail A, Abudayyeh A, Murakami N, Al-Shamsi HO, et al.. Immune checkpoint inhibitors for solid tumors in the adjuvant setting: current progress, future directions, and role in transplant oncology. Cancers. 2023;15(5). Epub 2023/03/12. doi: 10.3390/cancers15051433 ; PMCID: PMC10000896 - DOI - PMC - PubMed
-
- Lin JR, Chen YA, Campton D, Cooper J, Coy S, Yapp C, et al. High-plex immunofluorescence imaging and traditional histology of the same tissue section for discovering image-based biomarkers. Nat Cancer. 2023;4(7):1036–1052. Epub 2023/06/23. doi: 10.1038/s43018-023-00576-1 PMCID: PMC10368530. - DOI - PMC - PubMed
-
- Rigamonti A, Viatore M, Polidori R, Rahal D, Erreni M, Fumagalli MR, et al. Integrating ai-powered digital pathology and imaging mass cytometry identifies key classifiers of tumor cells, stroma, and immune cells in non–small cell lung cancer. Cancer Res. 2024;84(7):1165–1177. doi: 10.1158/0008-5472.CAN-23-1698 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

