Deciphering Auditory Hyperexcitability in Otogl Mutant Mice Unravels an Auditory Neuropathy Mechanism
- PMID: 39965080
- PMCID: PMC12097039
- DOI: 10.1002/advs.202410776
Deciphering Auditory Hyperexcitability in Otogl Mutant Mice Unravels an Auditory Neuropathy Mechanism
Abstract
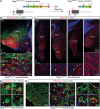
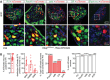
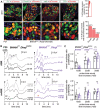
Auditory neuropathies affect the spiral ganglion neurons of the auditory nerve or their synapses with the sensory hair cells, distorting the sound information transmitted from the ear to the brain. Deciphering the underlying pathophysiological mechanisms remains challenging owing to the diversity of spiral ganglion neuron subtypes and associated central auditory circuits. An auditory neuropathy mechanism is unraveled by investigating the origin of auditory hyperexcitability in a mouse model for hereditary congenital deafness. Otogl encodes the large Otogelin-like protein, which is related to secreted epithelial mucins and is implicated in the mechanical stimulation of cochlear outer hair cells. Heterozygous Otogl+/- mutant mice display auditory hyperexcitability, highlighted by their susceptibility to audiogenic seizures induced by loud sounds. It is shown that Otogl is transiently expressed in a subpopulation of spiral ganglion neurons during cochlear development. Despite their apparently normal hearing, Otogl+/- mice display poor activation of the spiral ganglion neurons processing loud sounds and an elevation of the activation threshold of the middle the ear muscle reflex that attenuates loud sounds. The findings reveal how a neuropathy affecting spiral ganglion neurons specialized in loud sound processing and associated with the middle the ear muscle reflex can manifest itself as auditory hyperexcitability.
Keywords: Otogelin‐like; auditory neuropathy; low spontaneous rate spiral ganglion neurons; middle ear muscle reflex; reemerging auditory brainstem responses.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
C.P. and P.A. are members of the scientific advisory board of Sensorion.
Figures







References
-
- Giraudet F., Avan P., Curr. Opin. Neurol. 2012, 25, 50. - PubMed
-
- Starr A., Picton T. W., Sininger Y., Hood L. J., Berlin C. I., Brain J. Neurol. 1996, 119, 741. - PubMed
-
- Starr A., McPherson D., Patterson J., Don M., Luxford W., Shannon R., Sininger Y., Tonakawa L., Waring M., Brain J. Neurol. 1991, 114, 1157. - PubMed
-
- Kiang N. Y.‐S., Discharge Patterns Of Single Fibers In The Cat's Auditory Nerve, The MIT Press, Cambridge, MA, USA: 1965.
MeSH terms
Supplementary concepts
Grants and funding
- ANR-10-INSB-04-01/French Government Programme Investissements d'Avenir France BioImaging
- ANR-18-RHUS-0007/Agence Nationale de la Recherche "AUDINNOVE"
- ANR-10-LABX-62-IBEID/Laboratoire d'Excellence "Integrative Biology of Emerging Infectious Diseases"
- ANR-15-RHUS-0001/"LIGHT4DEAF"
- ANR-10-LABX-65/Agence Nationale de la Recherche "LIFESENSES"
- ANR-21-CE34-0012/Agence Nationale de la Recherche "FATIGAUDIT"
- ANR-23-CE17-0029/Agence Nationale de la Recherche "TIME-TO-EAR"
- ANR-23-IAHU-0003/Agence Nationale de la Recherche, the France 2030 program
- the Raimonde and Guy Strittmatter Foundation
- PTR618-23/the Institut Pasteur
- FPAIDA02/Fondation Pour l'Audition
- RD-2023-1/Fondation Pour l'Audition
- FPAIDA07/Fondation Pour l'Audition
- FPAIDA05/Fondation Pour l'Audition
- FPAIDA03/Fondation Pour l'Audition
- ALTF 852-2019/European Molecular Biology Organization
- H2020-MSCA-IF-2017/the Marie Skłodowska-Curie initiative
- FDM201806005994/the Fondation pour la Recherche Medicale
- Sorbonne Université PhD Program
- the Institut Pasteur - Paris University International Doctoral Program
LinkOut - more resources
Full Text Sources
