Enhancing Lung Recovery: Inhaled Poly(lactic- co-glycolic) Acid Encapsulating FTY720 and Nobiletin for Lipopolysaccharide-Induced Lung Injury, with Advanced Inhalation Tower Technology
- PMID: 39965088
- PMCID: PMC11887484
- DOI: 10.1021/acsnano.3c12532
Enhancing Lung Recovery: Inhaled Poly(lactic- co-glycolic) Acid Encapsulating FTY720 and Nobiletin for Lipopolysaccharide-Induced Lung Injury, with Advanced Inhalation Tower Technology
Abstract
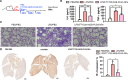
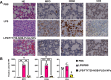
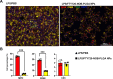
Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), a rapidly progressing respiratory failure condition, results in a high mortality rate, especially in severe cases. Numerous trials have investigated various pharmacotherapy approaches, but their effectiveness remains uncertain. Here, we present an inhaled nanoformulation of fingolimod (FTY720)-nobiletin (NOB)- poly(lactic-co-glycolic) acid (PLGA) nanoparticles (NPs) with good biocompatibility and a sustained-release pharmacological effect. The formulation decreases the toxicity of FTY720 and increases the bioavailability of NOB since we use PLGA with a high biocompatibility to encapsulate FTY720 and NOB at the same time. In vitro, in comparison to treatment with the pure drug, we demonstrated that FTY720-NOB-PLGA NPs can reduce interleukin-6 (IL-6) and reactive oxygen species (ROS) release by macrophages after lipopolysaccharide (LPS) stimulation more efficiently. In vivo, we used an inhalation tower system that allowed the exposure of unanesthetized mice to aerosolized FTY720-NOB-PLGA NPs under controlled conditions. We demonstrated that inhaled FTY720-NOB-PLGA NPs can attenuate lung injury after LPS exposure by suppressing cytokine release, such as IL-6 and tumor necrosis factor-α (TNF-α). The trigger pathway of ALI, including nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and p38 mitogen-activated protein kinase, was also efficiently inhibited. Furthermore, the inhalation treatment provided a good safety profile, without detrimental effects on biochemical markers and lung function. We provided the feasibility of administering inhalation of NPs noninvasively with continuous monitoring of lung function. The aerosolized FTY720-NOB-PLGA NPs we developed show excellent promise for acute lung injury therapy in the future.
Keywords: acute lung injury (ALI); cytokine suppression; fingolimod (FTY720); immune cell infiltration; inhaled nanoformulation; nobiletin (NOB).
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Bellani G.; Laffey J. G.; Pham T.; Fan E.; Brochard L.; Esteban A.; Gattinoni L.; van Haren F.; Larsson A.; McAuley D. F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. Jama 2016, 315 (8), 788–800. 10.1001/jama.2016.0291. - DOI - PubMed
-
From NLM.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

