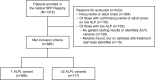
Disease burden by ALPL variant number in patients with non-life-threatening hypophosphatasia in the Global HPP Registry
- PMID: 39965917
- PMCID: PMC12015031
- DOI: 10.1136/jmg-2024-110383
Disease burden by ALPL variant number in patients with non-life-threatening hypophosphatasia in the Global HPP Registry
Abstract
Background: Hypophosphatasia (HPP) is a rare metabolic disease caused by autosomal dominant or recessive inheritance of ALPL variants resulting in low alkaline phosphatase activity. The objective of this analysis was to compare HPP disease burden between patients with non-life-threatening disease in the Global HPP Registry who have one ALPL variant versus two or more ALPL variants.
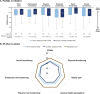
Methods: Patients were included if they had one or more ALPL variants identified through genetic testing and first HPP manifestations after 6 months of age. Assessments included history of HPP manifestations, Brief Pain Inventory-Short Form (BPI-SF), Health Assessment Questionnaire-Disability Index (HAQ-DI), 6-Min Walk Test (6MWT), Paediatric Quality of Life Inventory (PedsQL) and 36-Item Short-Form Survey V.2 (SF-36v2).
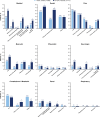
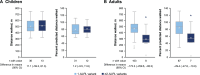
Results: Of 685 included patients, 568 (82.9%) had one ALPL variant, 116 (16.9%) had two variants, and one (0.1%) had three variants. Patients with two or more ALPL variants had higher proportions of skeletal (52.1% vs 32.6%), dental (73.5% vs 56.0%), muscular (36.8% vs 23.6%) and neurological (22.2% vs 8.8%) manifestations at last assessment. BPI-SF, HAQ-DI, PedsQL and SF-36v2 scores were similar between groups. Distances walked on the 6MWT were similar between groups for children. Distance walked was lower among adults with two or more variants (293 m (n=8)) than adults with one variant (466 m (n=103)), although the former group was very small.
Conclusion: HPP disease burden is high in patients with HPP, regardless of ALPL variant number. While prevalence of HPP-specific manifestations was higher in patients with two or more variants than those with one variant, patient-reported outcomes were similar between groups.
Trial registration number: NCT02306720; EUPAS13514.
Keywords: gene frequency; genetic association studies; genetic diseases, inborn.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: PSK, KMD, GÁM-M, AL, CRG, KO, WH and LS consult for/have received research funding/honoraria from Alexion, AstraZeneca Rare Disease. AP, WRM and SF are employees of and may own stock/options in Alexion, AstraZeneca Rare Disease. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
