Fluorogenic Platform for Real-Time Imaging of Subcellular Payload Release in Antibody-Drug Conjugates
- PMID: 39965918
- PMCID: PMC11887046
- DOI: 10.1021/jacs.4c16842
Fluorogenic Platform for Real-Time Imaging of Subcellular Payload Release in Antibody-Drug Conjugates
Abstract
Antibody-drug conjugates (ADCs) represent promising therapeutic constructs to enhance the selective delivery of drugs to target cells; however, attaining precise control over the timing and location of payload release remains challenging due to the complex intracellular processes that define ADC internalization, trafficking, and linker cleavage. In this study, we present novel real-time fluorogenic probes to monitor both subcellular dynamics of ADC trafficking and payload release. We optimized a tandem molecular design of sequential pH- and enzyme-activatable naphthalimide fluorophores to (1) track their subcellular localization along the endolysosomal pathway and (2) monitor linker cleavage with OFF-to-ON fluorescence switches. Live-cell imaging microscopy revealed that fluorogenic ADCs can traffic to the lysosomes and yet require residence time in these subcellular compartments for efficient linker cleavage. Notably, the compact size of fluorogenic naphthalimides did not impair the recognition of target cell surface reporters or the kinetics of payload release. This modular platform is applicable to many ADCs and holds promise to inform their rational design for optimal release profiles and therapeutic efficacy.
Conflict of interest statement
The authors declare the following competing financial interest(s): P.L.S., K.A.S, Z.W., R.D.W., V.L.M., and M.J.M. are employees of AbbVie. C.C.M. and A.D.H. were employees of AbbVie at the time of the study. AbbVie contributed in part to the design, study conduct, and financial support for this research.
Figures
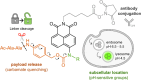

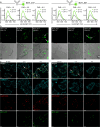


References
-
- Bross P. F.; Beitz J.; Chen G.; Chen X. H.; Duffy E.; Kieffer L.; Roy S.; Sridhara R.; Rahman A.; Williams G.; et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 2001, 7, 1490–1496. - PubMed
-
- Mosele F.; Deluche E.; Lusque A.; Le Bescond L.; Filleron T.; Pradat Y.; Ducoulombier A.; Pistilli B.; Bachelot T.; Viret F.; et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: the phase 2 DAISY trial. Nat. Med. 2023, 29, 2110–2120. 10.1038/s41591-023-02478-2. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

