Mitochondrial alterations and signatures in hepatocellular carcinoma
- PMID: 39966277
- PMCID: PMC11836208
- DOI: 10.1007/s10555-025-10251-9
Mitochondrial alterations and signatures in hepatocellular carcinoma
Abstract
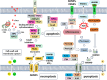
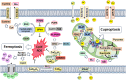
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer worldwide. Its primary risk factors are chronic liver diseases such as metabolic fatty liver disease, non-alcoholic steatohepatitis, and hepatitis B and C viral infections. These conditions contribute to a specific microenvironment in liver tumors which affects mitochondrial function. Mitochondria are energy producers in cells and are responsible for maintaining normal functions by controlling mitochondrial redox homeostasis, metabolism, bioenergetics, and cell death pathways. HCC involves abnormal mitochondrial functions, such as accumulation of reactive oxygen species, oxidative stress, hypoxia, impairment of the mitochondrial unfolded protein response, irregularities in mitochondrial dynamic fusion/fission mechanisms, and mitophagy. Cell death mechanisms, such as necroptosis, pyroptosis, ferroptosis, and cuproptosis, contribute to hepatocarcinogenesis and play a significant role in chemoresistance. The relationship between mitochondrial dynamics and HCC is thus noteworthy. In this review, we summarize the recent advances in mitochondrial alterations and signatures in HCC and attempt to elucidate its molecular biology. Here, we provide an overview of the mitochondrial processes involved in hepatocarcinogenesis and offer new insights into the molecular pathology of the disease. This may help guide future research focused on improving patient outcomes using innovative therapies.
Keywords: Chemoresistance; Hepatocarcinogenesis; Hepatocellular carcinoma; Mitochondria; Mitochondrial dynamics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Villanueva, A. (2019). Hepatocellular carcinoma. New England Journal of Medicine,380(15), 1450–1462. 10.1056/NEJMra1713263 - PubMed
-
- Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians,71(3), 209–249. 10.3322/caac.21660 - PubMed
-
- Ben-Moshe, S., & Itzkovitz, S. (2019). Spatial heterogeneity in the mammalian liver. Nature Reviews. Gastroenterology & Hepatology,16(7), 395–410. 10.1038/s41575-019-0134-x - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

