Eukaryotic Elongation Factor 2 Kinase EFK-1/eEF2K promotes starvation resistance by preventing oxidative damage in C. elegans
- PMID: 39966347
- PMCID: PMC11836464
- DOI: 10.1038/s41467-025-56766-1
Eukaryotic Elongation Factor 2 Kinase EFK-1/eEF2K promotes starvation resistance by preventing oxidative damage in C. elegans
Abstract
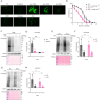
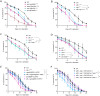
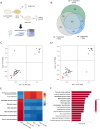
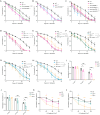
Cells and organisms frequently experience starvation. To survive, they mount an evolutionarily conserved stress response. A vital component in the mammalian starvation response is eukaryotic elongation factor 2 (eEF2) kinase (eEF2K), which suppresses translation in starvation by phosphorylating and inactivating the translation elongation driver eEF2. C. elegans EFK-1/eEF2K phosphorylates EEF-2/eEF2 on a conserved residue and is required for starvation survival, but how it promotes survival remains unclear. Surprisingly, we found that eEF2 phosphorylation is unchanged in starved C. elegans and EFK-1's kinase activity is dispensable for starvation survival, suggesting that efk-1 promotes survival via a noncanonical pathway. We show that efk-1 upregulates transcription of DNA repair pathways, nucleotide excision repair (NER) and base excision repair (BER), to promote starvation survival. Furthermore, efk-1 suppresses oxygen consumption and ROS production in starvation to prevent oxidative stress. Thus, efk-1 enables starvation survival by protecting animals from starvation-induced oxidative damage through an EEF-2-independent pathway.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: JHH is a paid consultant for Surrozen, Inc. All other authors declare no competing interests.
Figures




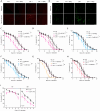
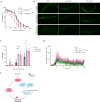
References
-
- El-Naggar, A. M. & Sorensen, P. H. Translational control of aberrant stress responses as a hallmark of cancer. J. Pathol.244, 650–666 (2018). - PubMed
-
- Delaidelli, A. et al. TB-16 exploration of eEF2K as a novel therapeutic target in medulloblastoma and neuroblastoma. Neuro-Oncol18, iii171–iii171 (2016).
MeSH terms
Substances
Grants and funding
- USRA studentship/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- R00 ES029552/ES/NIEHS NIH HHS/United States
- P40 OD010440/OD/NIH HHS/United States
- RGPIN-2024-06537/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- CGS-D scholarship/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
- IGAP/BC Children's Hospital Foundation (British Columbia Children's Hospital Foundation)
- ALLRP 597082-24/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- Studentship/BC Children's Hospital Foundation (British Columbia Children's Hospital Foundation)
- FDN-143280/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

