Cooperation between the Hippo and MAPK pathway activation drives acquired resistance to TEAD inhibition
- PMID: 39966375
- PMCID: PMC11836325
- DOI: 10.1038/s41467-025-56634-y
Cooperation between the Hippo and MAPK pathway activation drives acquired resistance to TEAD inhibition
Abstract
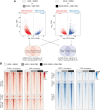
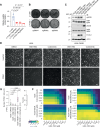
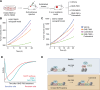
TEAD (transcriptional enhanced associate domain) transcription factors (TEAD1-4) serve as the primary effectors of the Hippo signaling pathway in various cancers. Targeted therapy leads to the emergence of resistance and the underlying mechanism of resistance to TEAD inhibition in cancers is less characterized. We uncover that upregulation of the AP-1 (activator protein-1) transcription factors, along with restored YAP (yes-associated protein) and TEAD activity, drives resistance to GNE-7883, a pan-TEAD inhibitor. Acute GNE-7883 treatment abrogates YAP-TEAD binding and attenuates FOSL1 (FOS like 1) activity. TEAD inhibitor resistant cells restore YAP and TEAD chromatin occupancy, acquire additional FOSL1 binding and exhibit increased MAPK (mitogen-activated protein kinase) pathway activity. FOSL1 is required for the chromatin binding of YAP and TEAD. This study describes a clinically relevant interplay between the Hippo and MAPK pathway and highlights the key role of MAPK pathway inhibitors in mitigating resistance to TEAD inhibition in Hippo pathway dependent cancers.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: All authors are or were employed by Genentech Inc., South San Francisco, CA, USA, at the time of their contributions to this work. All authors are or were shareholders at Roche except S.P., A.D.G., C. L., D.A.H.N.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

