Clusterin inhibits lipopolysaccharide induced liver injury
- PMID: 39966409
- PMCID: PMC11836311
- DOI: 10.1038/s41598-024-80903-3
Clusterin inhibits lipopolysaccharide induced liver injury
Abstract
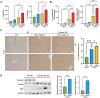
Lipopolysaccharide (LPS) exacerbates liver injury by activating various inflammatory pathways. Clusterin, a glycoprotein involved in lipid transport, and cytoprotection, is known to have inhibitory effects on liver steatosis and fibrosis. In this study, we investigated the role of clusterin in regulating LPS-induced liver injury and its effects on liver injury in C57BL/6 mice and clusterin knockout mice injected with LPS for 3 h. Primary Kupffer cells (KCs) and hepatocytes (HCs) were isolated from these mice and examined using immunohistochemistry, real-time RT-PCR, ELISA, and western blot analysis to assess the effects of clusterin. Clusterin deficiency significantly exacerbated LPS-induced liver injury, as evidenced by increased inflammatory cell infiltration, elevated serum alanine aminotransferase and aspartate aminotransferase levels, and upregulated expression of pro-inflammatory cytokines and components of the NLRP3 inflammasome. By contrast, overexpression of clusterin in primary Kupffer cells and hepatocytes significantly reduced these inflammatory markers. Furthermore, the protective mechanism of clusterin involved inhibition of the STAT3 signaling pathway. These findings suggest that clusterin is a useful therapeutic target to modulate cytokine production and key inflammatory signaling pathways in inflammatory liver diseases.
Keywords: Clusterin; LPS; Liver inflammation; NLRP3 inflammasome; STAT3.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





References
-
- Wu, Z., Han, M., Chen, T., Yan, W. & Ning, Q. Acute liver failure: mechanisms of immune-mediated liver injury. Liver Int.30, 782–794. 10.1111/j.1478-3231.2010.02262.x (2010). - PubMed
-
- Ghanim, H. et al. Increase in plasma endotoxin concentrations and the expression of toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 32, 2281–2287. 10.2337/dc09-0979 (2009). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

