Macrophage SUCLA2 coupled glutaminolysis manipulates obesity through AMPK
- PMID: 39966410
- PMCID: PMC11836283
- DOI: 10.1038/s41467-025-57044-w
Macrophage SUCLA2 coupled glutaminolysis manipulates obesity through AMPK
Abstract
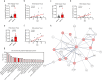
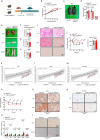
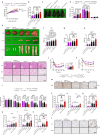
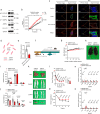
Obesity is regarded as a chronic inflammatory disease involving adipose tissue macrophages (ATM), but whether immunometabolic reprogramming of ATM affects obesity remains unclarified. Here we show that in ATM glutaminolysis is the fundamental metabolic flux providing energy and substrate, bridging with AMP-activated protein kinase (AMPK) activity, succinate-induced interleukin-1β (IL-1β) production, and obesity. Abrogation of AMPKα in myeloid cells promotes proinflammatory ATM, impairs thermogenesis and energy expenditure, and aggravates obesity in mice fed with high-fat diet (HFD). Conversely, IL-1β neutralization or myeloid IL-1β abrogation prevents obesity caused by AMPKα deficiency. Mechanistically, ATP generated from glutaminolysis suppresses AMPK to decrease phosphorylation of the β subunit of succinyl-CoA synthetase (SUCLA2), thereby resulting in the activation of succinyl-CoA synthetase and the overproduction of succinate and IL-1β; by contrast, siRNA-mediated SUCLA2 knockdown reduces obesity induced by HFD in mice. Lastly, phosphorylated SUCLA2 in ATM correlates negatively with obesity in humans. Our results thus implicate a glutaminolysis/AMPK/SUCLA2/IL-1β axis of inflammation and obesity regulation in ATM.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interest.
Figures


References
-
- Chooi, Y. C., Ding, C. & Magkos, F. The epidemiology of obesity. Metabolism92, 6–10 (2019). - PubMed
-
- Chavakis, T., Alexaki, V. I. & Ferrante, A. W. Jr Macrophage function in adipose tissue homeostasis and metabolic inflammation. Nat. Immunol.24, 757–766 (2023). - PubMed
-
- Sakamoto, T. et al. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. Am. J. Physiol.-Endocrinol. Metab.310, E676–E687 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

