GMRSP encoded by lncRNA H19 regulates metabolic reprogramming and alleviates aortic dissection
- PMID: 39966416
- PMCID: PMC11836370
- DOI: 10.1038/s41467-025-57011-5
GMRSP encoded by lncRNA H19 regulates metabolic reprogramming and alleviates aortic dissection
Abstract
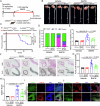
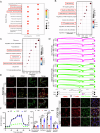
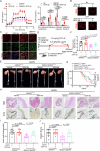
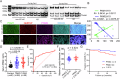
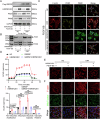
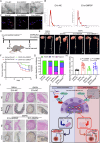
Metabolic disturbances are hallmarks of vascular smooth muscle cell (VSMC) phenotypic transitions, which play a critical role in the pathogenesis of aortic dissection (AD). In this study, we identify and characterize glucose metabolism regulatory protein (GMRSP), a protein encoded by lncRNA H19. Using VSMC-specific GMRSP induction in knock-in mice, adeno-associated virus-mediated GMRSP overexpression, and exosomal GMRSP delivery, we demonstrate significant improvements in AD and mitochondrial dysfunction. Mechanistically, GMRSP inhibits heterogeneous nuclear ribonucleoprotein (hnRNP) A2B1-mediated alternative splicing of pyruvate kinase M (PKM) pre-mRNA, leading to reduced PKM2 production and glycolysis. This reprogramming preserves the contractile phenotype of VSMCs and prevents their transition to a proliferative state. Importantly, pharmacological activation of PKM2 via TEPP-46 abrogates the protective effects of GMRSP in vivo and in vitro. Clinical relevance is shown by elevated plasma PKM2 levels in AD patients, which correlate with poor prognosis. Collectively, these findings indicate GMRSP as a key regulator of VSMC metabolism and phenotypic stability, highlighting its potential as a therapeutic target for AD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Evangelista, A. et al. Insights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation137, 1846–1860 (2018). - PubMed
-
- Ali, L., Schnitzler, J. G. & Kroon, J. Metabolism: The road to inflammation and atherosclerosis. Curr. Opin. Lipido.29, 474–480 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

