A postzygotic GNA13 variant upregulates the RHOA/ROCK pathway and alters melanocyte function in a mosaic skin hypopigmentation syndrome
- PMID: 39966435
- PMCID: PMC11836271
- DOI: 10.1038/s41467-025-56995-4
A postzygotic GNA13 variant upregulates the RHOA/ROCK pathway and alters melanocyte function in a mosaic skin hypopigmentation syndrome
Abstract
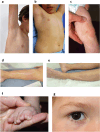
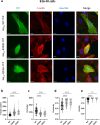
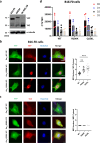
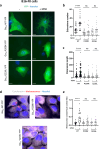
The genetic bases of mosaic pigmentation disorders have increasingly been identified, but these conditions remain poorly characterised, and their pathophysiology is unclear. Here, we report in four unrelated patients that a recurrent postzygotic mutation in GNA13 is responsible for a recognizable syndrome with hypomelanosis of Ito associated with developmental anomalies. GNA13 encodes Gα13, a subunit of αβγ heterotrimeric G proteins coupled to specific transmembrane receptors known as G-protein coupled receptors. In-depth functional investigations revealed that this R200K mutation provides a gain of function to Gα13. Mechanistically, we show that this variant hyperactivates the RHOA/ROCK signalling pathway that consequently increases actin polymerisation and myosin light chains phosphorylation, and promotes melanocytes rounding. Our results also indicate that R200K Gα13 hyperactivates the YAP signalling pathway. All these changes appear to affect cell migration and adhesion but not the proliferation. Our results suggest that hypopigmentation can result from a defect in melanosome transfer to keratinocytes due to cell shape alterations. These findings highlight the interaction between heterotrimeric G proteins and the RHOA pathway, and their role in melanocyte function.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
The GNA13-RhoA signaling axis suppresses expression of tumor protective Kallikreins.Cell Signal. 2016 Oct;28(10):1479-88. doi: 10.1016/j.cellsig.2016.07.001. Epub 2016 Jul 14. Cell Signal. 2016. PMID: 27424208
-
Inactivating mutations in GNA13 and RHOA in Burkitt's lymphoma and diffuse large B-cell lymphoma: a tumor suppressor function for the Gα13/RhoA axis in B cells.Oncogene. 2016 Jul 21;35(29):3771-80. doi: 10.1038/onc.2015.442. Epub 2015 Nov 30. Oncogene. 2016. PMID: 26616858 Free PMC article.
-
The interaction of Gα13 with integrin β1 mediates cell migration by dynamic regulation of RhoA.Mol Biol Cell. 2015 Oct 15;26(20):3658-70. doi: 10.1091/mbc.E15-05-0274. Epub 2015 Aug 26. Mol Biol Cell. 2015. PMID: 26310447 Free PMC article.
-
A novel pathogenic RHOA variant in a patient with patterned cutaneous hypopigmentation associated with extracutaneous findings.Pediatr Dermatol. 2022 Mar;39(2):281-287. doi: 10.1111/pde.14923. Epub 2022 Feb 17. Pediatr Dermatol. 2022. PMID: 35178721 Free PMC article.
-
Rho kinase and hypertension.Biochim Biophys Acta. 2010 Dec;1802(12):1276-84. doi: 10.1016/j.bbadis.2010.05.002. Epub 2010 May 9. Biochim Biophys Acta. 2010. PMID: 20460153 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

