Aβ42 induces stress granule formation via PACT/PKR pathway
- PMID: 39966464
- PMCID: PMC11836309
- DOI: 10.1038/s41598-025-88380-y
Aβ42 induces stress granule formation via PACT/PKR pathway
Abstract
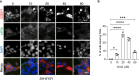
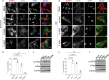
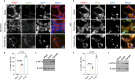
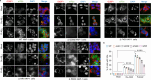
Stress granule (SG) formation has been linked to several neurodegenerative disorders, such as Alzheimer's disease (AD). Amyloid-β42 (Aβ42) is a key player in the pathogenesis of AD and is known to trigger various stress-related signaling pathways. However, the impact of Aβ on SG formation has not been fully understood. The primary aim of this study is to analyze the SG-inducing properties of Aβ42 and to uncover the molecular mechanisms underlying this process. Our results revealed that exposure to 20 μM Aβ42 led to a significant SG formation in neuroblastoma-derived (SH-SY5Y) and glioma-derived (U87) cell lines. Interestingly, we observed elevated levels of p-eIF2α, while overall protein translation levels remained unchanged. Monomeric and oligomeric forms of Aβ42 exhibited a 4-5 times stronger ability to induce SG formation compared to fibrillar forms. Additionally, treatment with familial mutants of Aβ42 (Dutch and Flemish) showed distinct effects on SG induction. Moreover, our findings using eIF2α kinases knockout (KO) cell lines demonstrated that Aβ-induced SG formation is primarily dependent on Protein Kinase R (PKR). Subsequent proximity ligation assay (PLA) analysis revealed a close proximity of PACT and PKR in Aβ-treated cells and in AD mouse hippocampus. Taken together, our study suggests that Aβ42 promotes SG formation through PKR kinase activation, which in turn requires PACT involvement.
Keywords: Alzheimer’s; Amyloid-β; Aβ42; PKR kinase; Stress granule; Stress signaling.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

