Parent-of-origin testing of prenatal copy number variations: a retrospective study of 167 family cases
- PMID: 39966468
- PMCID: PMC11836297
- DOI: 10.1038/s41598-025-86487-w
Parent-of-origin testing of prenatal copy number variations: a retrospective study of 167 family cases
Abstract
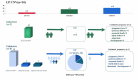
Low-penetrance pathogenic copy number variations (CNVs), variation of uncertain significance (VUS) CNVs and likely pathogenic CNVs present a challenge for prenatal diagnosis. Previous studies have clarified the influence of a parent-of-origin test on the prenatal VUS CNVs. However, the influence of parent-of-origin tests on prenatal likely pathogenic (LP) or low-penetrance pathogenic CNVs (pCNVs) have not been evaluated. Here, among 2273 pregnant women undergoing prenatal diagnosis, 236 CNVs were reported by chromosomal microarray analysis (CMA) including 69 full-penetrance pCNVs, 44 low-penetrance pCNVs, 113 VUS CNVs and 10 LP CNVs. Based on the subsequent parent-of-origin tests, CNVs were classified as de novo, inherited and unknown group. Firstly, a total of 112 couple (62 VUS CNVs, two LP CNVs and 48 pCNVs) chose parent-of-origin tests and 88 inherited CNVs (51 VUS CNVs, two LP CNVs and 35 pCNVs ) were identified. Then, the effect of parent-of-origin tests was focused on 44 low-penetrance pCNVs, 113 VUS CNVs and 10 LP CNVs in this study (n = 167). For 44 low-penetrance pCNVs, termination of pregnancy (TOP) rates in de novo, inherited and unknown group were 100% (5/5), 23.5% (4/17) and 40.9% (9/22), respectively. TOP decisions in low-penetrance pCNVs were mainly affected by de novo and abnormal ultrasound findings. For 113 VUS CNVs, inherited VUS CNVs dramatically reduced anxiety reflected by TOP rates in de novo (18.2%, 2/11), inherited (0/51) and unknown group (2.0%, 1/51). Notably, prenatal minor structural defects often disappeared after birth. These results suggested the majority VUS CNVs have no appreciable pathogenicity. For 10 LP CNVs, TOP rates in inherited and unknown group were 0% (0/2) and 87.5% (7/8), which suggested that it is imperative that parent-of-origin tests be offered for LP CNVs to bring the classification to pathogenic or VUS.
Keywords: Copy number variations; Likely pathogenicity; Low-penetrance pathogenicity; Parent-of-origin test; Variation of uncertain significance.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
[Prenatal utility of parental source verification on the interpretation of copy number variation identified by chromosomal microarray analysis].Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2022 May 10;39(5):542-545. doi: 10.3760/cma.j.cn511374-20210122-00066. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2022. PMID: 35598275 Chinese.
-
Detection of copy number variants using chromosomal microarray analysis for the prenatal diagnosis of congenital heart defects with normal karyotype.J Clin Lab Anal. 2019 Jan;33(1):e22630. doi: 10.1002/jcla.22630. Epub 2018 Jul 25. J Clin Lab Anal. 2019. PMID: 30047171 Free PMC article.
-
The uncertainty of copy number variants: pregnancy decisions and clinical follow-up.Am J Obstet Gynecol. 2023 Aug;229(2):170.e1-170.e8. doi: 10.1016/j.ajog.2023.01.022. Epub 2023 Jan 27. Am J Obstet Gynecol. 2023. PMID: 36716986
-
The impact of human copy number variation on a new era of genetic testing.BJOG. 2010 Mar;117(4):391-8. doi: 10.1111/j.1471-0528.2009.02470.x. Epub 2010 Jan 26. BJOG. 2010. PMID: 20105165 Review.
-
Chromosome copy number variants in fetuses with syndromic malformations.Birth Defects Res. 2017 Jun 1;109(10):725-733. doi: 10.1002/bdr2.1054. Birth Defects Res. 2017. PMID: 28568742 Review.
References
-
- Riggs, E. R. et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet. Med.22, 245–257. 10.1038/s41436-019-0686-8 (2020). - PMC - PubMed
-
- Portnoi, M. F. Microduplication 22q11.2: A new chromosomal syndrome. Eur. J. Med. Genet.52, 88–93. 10.1016/j.ejmg.2009.02.008 (2009). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

