Cyclic mechanical loading of photopolymerized methacrylated hydrogels for probing interdependent effects of strain, stiffness, and substrate composition in pulmonary fibrogenesis
- PMID: 39966483
- PMCID: PMC11836278
- DOI: 10.1038/s41598-025-90753-2
Cyclic mechanical loading of photopolymerized methacrylated hydrogels for probing interdependent effects of strain, stiffness, and substrate composition in pulmonary fibrogenesis
Abstract
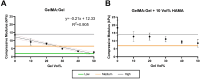
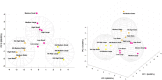
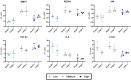
Pulmonary fibrosis is characterized by excessive deposition of extracellular matrix (ECM), stiffening of the lung tissue, and impaired gas exchange. Our current understanding of fibrogenesis generally focuses on the individual roles of mechanical and biochemical stimuli in driving disease progression. However, many mechano-chemical pathways are interrelated, so dissecting the interactive effects of mechanical and biochemical signals is an important knowledge gap. To address this gap, we investigated lung fibroblast behavior on static and cyclically strained photopolymerizable hydrogels consisting of different ratios of methacrylated gelatin, methacrylated hyaluronan, and non-methacrylated gelatin to create substrates with tunable stiffness and chemistry, representative of both healthy and fibrotic lung ECM properties. We observed that higher stiffness gels amplified the impact of strain, resulting in distinct differences in expression of MMP1, CTGF, Rho/ROCK, and ECM deposition genes. Substrates with hyaluronan demonstrated a capacity to modulate strain-induced fibrogenic responses, suggesting a buffering effect of hyaluronan on fibrotic disease progression. Overall, our results highlight mechanotransductive changes in gene expression in response to substrate composition, stiffness, and cyclic mechanical strain. Through the controlled study of mechanical and biochemical cues, our findings contribute to a deeper understanding of the pathogenesis of pulmonary fibrosis.
Keywords: Fibrogenic response; GelMA; HAMA; In vitro lung model; Mechanobiology.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications.Int J Mol Sci. 2021 Jun 23;22(13):6758. doi: 10.3390/ijms22136758. Int J Mol Sci. 2021. PMID: 34201769 Free PMC article.
-
An in vitro model of fibrosis using crosslinked native extracellular matrix-derived hydrogels to modulate biomechanics without changing composition.Acta Biomater. 2022 Jul 15;147:50-62. doi: 10.1016/j.actbio.2022.05.031. Epub 2022 May 21. Acta Biomater. 2022. PMID: 35605955
-
Pulmonary Stretch and Lung Mechanotransduction: Implications for Progression in the Fibrotic Lung.Int J Mol Sci. 2021 Jun 16;22(12):6443. doi: 10.3390/ijms22126443. Int J Mol Sci. 2021. PMID: 34208586 Free PMC article. Review.
-
Single-cell transcriptome analysis revealing mechanotransduction via the Hippo/YAP pathway in promoting fibroblast-to-myofibroblast transition and idiopathic pulmonary fibrosis development.Gene. 2025 Apr 5;943:149271. doi: 10.1016/j.gene.2025.149271. Epub 2025 Jan 22. Gene. 2025. PMID: 39855369
-
The extracellular matrix and mechanotransduction in pulmonary fibrosis.Int J Biochem Cell Biol. 2020 Sep;126:105802. doi: 10.1016/j.biocel.2020.105802. Epub 2020 Jul 12. Int J Biochem Cell Biol. 2020. PMID: 32668329 Review.
References
-
- Wen, D. S. et al. Focusing on Mechanoregulation Axis in Fibrosis: Sensing, Transduction and Effecting. Front Mol Biosci 9 (2022). https://doi.org/ARTN 80468010.3389/fmolb.2022.804680. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

