Archaean green-light environments drove the evolution of cyanobacteria's light-harvesting system
- PMID: 39966498
- PMCID: PMC11976284
- DOI: 10.1038/s41559-025-02637-3
Archaean green-light environments drove the evolution of cyanobacteria's light-harvesting system
Abstract
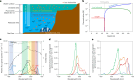
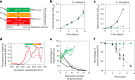
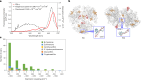
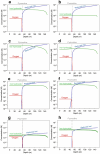
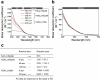
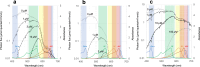
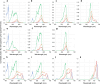
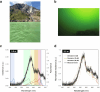
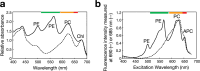
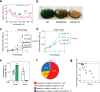
Cyanobacteria induced the great oxidation event around 2.4 billion years ago, probably triggering the rise in aerobic biodiversity. While chlorophylls are universal pigments used by all phototrophic organisms, cyanobacteria use additional pigments called phycobilins for their light-harvesting antennas-phycobilisomes-to absorb light energy at complementary wavelengths to chlorophylls. Nonetheless, an enigma persists: why did cyanobacteria need phycobilisomes? Here, we demonstrate through numerical simulations that the underwater light spectrum during the Archaean era was probably predominantly green owing to oxidized Fe(III) precipitation. The green-light environments, probably shaped by photosynthetic organisms, may have directed their own photosynthetic evolution. Genetic engineering of extant cyanobacteria, simulating past natural selection, suggests that cyanobacteria that acquired a green-specialized phycobilin called phycoerythrobilin could have flourished under green-light environments. Phylogenetic analyses indicate that the common ancestor of modern cyanobacteria embraced all key components of phycobilisomes to establish an intricate energy transfer mechanism towards chlorophylls using green light and thus gained strong selective advantage under green-light conditions. Our findings highlight the co-evolutionary relationship between oxygenic phototrophs and light environments that defined the aquatic landscape of the Archaean Earth and envision the green colour as a sign of the distinct evolutionary stage of inhabited planets.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Diverse Chromatic Acclimation Processes Regulating Phycoerythrocyanin and Rod-Shaped Phycobilisome in Cyanobacteria.Mol Plant. 2019 May 6;12(5):715-725. doi: 10.1016/j.molp.2019.02.010. Epub 2019 Feb 26. Mol Plant. 2019. PMID: 30818037
-
Phycourobilin in trichromatic phycocyanin from oceanic cyanobacteria is formed post-translationally by a phycoerythrobilin lyase-isomerase.J Biol Chem. 2009 Apr 3;284(14):9290-8. doi: 10.1074/jbc.M809784200. Epub 2009 Jan 31. J Biol Chem. 2009. PMID: 19182270 Free PMC article.
-
Cyanobacterial photosynthesis in the oceans: the origins and significance of divergent light-harvesting strategies.Trends Microbiol. 2002 Mar;10(3):134-42. doi: 10.1016/s0966-842x(02)02319-3. Trends Microbiol. 2002. PMID: 11864823 Review.
-
Characterization of Molecular Diversity and Organization of Phycobilisomes in Thermophilic Cyanobacteria.Int J Mol Sci. 2023 Mar 15;24(6):5632. doi: 10.3390/ijms24065632. Int J Mol Sci. 2023. PMID: 36982707 Free PMC article.
-
Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis.Curr Opin Plant Biol. 2013 Jun;16(3):307-14. doi: 10.1016/j.pbi.2013.03.011. Epub 2013 Apr 11. Curr Opin Plant Biol. 2013. PMID: 23583332 Review.
References
-
- Sagan, C. Pale Blue Dot: A Vision of the Human Future in Space (Random House, 1994).
-
- Payne, J. L. et al. The evolutionary consequences of oxygenic photosynthesis: a body size perspective. Photosynth. Res.107, 37–57 (2011). - PubMed
-
- Jabłońska, J. & Tawfik, D. S. The evolution of oxygen-utilizing enzymes suggests early biosphere oxygenation. Nat. Ecol. Evol.5, 442–448 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

