Intraductal chemotherapy for triple-negative breast cancer: a pathway to minimally invasive clinical treatment
- PMID: 39966717
- PMCID: PMC11837698
- DOI: 10.1186/s12885-025-13693-0
Intraductal chemotherapy for triple-negative breast cancer: a pathway to minimally invasive clinical treatment
Abstract
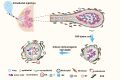
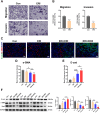
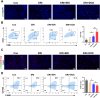
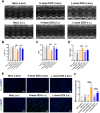
Triple-negative breast cancer (TNBC) is traditionally treated with systemic chemotherapy, often resulting in significant off-target toxicity. In this study, we assess the efficacy of intraductal chemotherapeutic delivery, aimed at reducing systemic side effects. Using an in situ TNBC model, created by intraductal injection of 4T1-luc cells, we identified day 3 post-tumor implantation as an optimal early intervention point. Echocardiographic analysis confirmed that intraductal administration of eribulin (ERI) or doxorubicin (DOX) did not cause cardiac dysfunction or apoptosis. Our results demonstrate that intraductal delivery of ERI and DOX significantly enhances anti-tumor and anti-metastatic effects. Mechanistically, ERI followed by DOX increased intratumoral perfusion, improved drug concentration, reversed epithelial-mesenchymal transition, and inhibited tumor cell invasion and metastasis. Additionally, this approach triggered immunogenic cell death and activated a systemic anti-tumor immune response. These findings underscore the potential of intraductal chemotherapy as a safe, highly effective approach, offering a preclinical foundation for minimally invasive TNBC therapies.
Keywords: Chemo-immunomodulation; Epithelial-mesenchymal transition; Intraductal therapy; Minimally invasive treatment; Triple-negative breast cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The People’s Hospital of Wuhan University’s Animal Ethics Committee approved this round of animal testing (IACUC Issue No. 20200702), and all ethical guidelines were strictly followed. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer. BMJ. 2023;381:e071674. - PubMed
-
- Wang G, Kumar A, Ding W, Korangath P, Bera T, Wei J, Pai P, Gabrielson K, Pastan I, Sukumar S. Intraductal administration of transferrin receptor-targeted immunotoxin clears ductal carcinoma in situ in mouse models of breast cancer-a preclinical study. Proc Natl Acad Sci U S A. 2022;119(24):e2200200119. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- CXPJJH123001-2320/the Hubei Chen Xiaoping Science and Technology Development Foundation Youth Science Special Fund
- 2022SWZX09/Subject of Biomedical Research Center of Hubei Cancer Hospital
- 2042019kf0229/the Fundamental Research Funds for the Central Universities
- 81802895/the National Natural Science Foundation of China
- 2023AFB493/the Natural Science Foundation of Hubei Province, China
LinkOut - more resources
Full Text Sources

