Early cytokine signatures and clinical phenotypes discriminate persistent from resolving MRSA bacteremia
- PMID: 39966757
- PMCID: PMC11834594
- DOI: 10.1186/s12879-025-10620-3
Early cytokine signatures and clinical phenotypes discriminate persistent from resolving MRSA bacteremia
Abstract
Background: Staphylococcus aureus bacteremia (SAB) is a prevalent life-threatening infection often caused by methicillin-resistant S. aureus (MRSA). Up to 30% of SAB patients fail to clear infection even with gold-standard anti-MRSA antibiotics. This phenomenon is termed antibiotic-persistent MRSA bacteremia (APMB). The mechanisms driving APMB are complex and involve host phenotypes significantly impacting the immune response. Thus, defining early immune signatures and clinical phenotypes that differentiate APMB from antibiotic resolving (AR)MB could aid therapeutic success.
Methods: We assessed 38 circulating cytokines and chemokines using affinity proteomics in 74 matched pairs of vancomycin-treated SAB cases identified as ARMB or APMB after 5 days of blood culture.
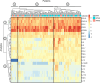
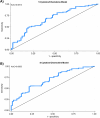
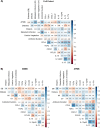
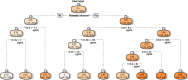
Results: Unsupervised hierarchical clustering segregated APMB from ARMB based on differential levels of IL-10, IL-12p40, IL-13, CCL4, and TGFα. Additionally, CXCL1, CCL22 and IL-17A significantly differed between APMB and ARMB when correlated with diabetes, dialysis, metastatic infection, or cardiac vegetation. Combining immune signatures with these relevant clinical phenotypes sharply increased accuracy of discriminating APMB outcome to 79.1% via logistic regression modeling. Finally, classification-regression tree analysis revealed explicit analyte thresholds associated with APMB outcome at presentation especially in patients with metastatic infection.
Conclusions: Collectively, this study identifies previously unrecognized cytokine and chemokine signatures that distinguish APMB and ARMB at presentation and in the context of host clinical characteristics associated with increased disease severity. Validation of a biomarker signature that accurately predicts outcomes could guide early therapeutic strategies and interventions to reduce risks of persistent SAB that are associated with worsened morbidity and mortality.
Keywords: Staphylococcus aureus; Clinical phenotypes; Cytokine signatures; MRSA; Persistence.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with Good Clinical Practice and Human Subjects Research as previously approved by the Duke University Medical Center (DUMC) Institutional Review Board (Approval Number: Pro00008031). The patients for this study were selected from and prospectively enrolled via an informed consent process under the Duke Institutional Review Board (IRB) Protocol # Pro00008031 between 2007 and 2017. If a patient died, written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Consent for publication: Not applicable. Competing interests: VGF reports Grant/ Research Support: MedImmune, Cerexa/Forest/Actavis/Allergan, Pfizer, Advanced Liquid Logics, Theravance, Novartis, Cubist/Merck; Medical Biosurfaces; Locus; Affinergy; Contrafect; Karius; Genentech, Regeneron, BasileaPaid Consultant: Pfizer, Novartis, Galderma, Novadigm, Durata, Debiopharm, Genentech, Achaogen, Affinium, Medicines Co., Cerexa, Tetraphase, Trius, MedImmune, Bayer, Theravance, Cubist, Basilea, Affinergy, Janssen, xBiotech, Contrafect, Regeneron, Basilea, Destiny. Membership: Merck Co-Chair V710 Vaccine. Educational fees: Green Cross, Cubist, Cerexa, Durata, Theravance; Debiopharm. Royalties: UpToDate.M.R.Y is a founder and shareholder of NovaDigm Therapeutics, Inc. which develops anti-infective vaccines and immunotherapies targeting S. aureus and other pathogens. He holds patents on anti-infectives, vaccines and immunotherapeutics targeting infectious diseases, including those caused by S. aureus.
Figures


References
-
- Laupland KB, Lyytikainen O, Sogaard M, Kennedy KJ, Knudsen JD, Ostergaard C, et al. The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect. 2013;19(5):465–71. - PubMed
-
- Tom S, Galbraith JC, Valiquette L, Jacobsson G, Collignon P, Schonheyder HC, et al. Case fatality ratio and mortality rate trends of community-onset Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2014;20(10):O630–2. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

