Integration of single-cell and spatial transcriptomics reveals fibroblast subtypes in hepatocellular carcinoma: spatial distribution, differentiation trajectories, and therapeutic potential
- PMID: 39966876
- PMCID: PMC11837652
- DOI: 10.1186/s12967-025-06192-0
Integration of single-cell and spatial transcriptomics reveals fibroblast subtypes in hepatocellular carcinoma: spatial distribution, differentiation trajectories, and therapeutic potential
Abstract
Background: Cancer-associated fibroblasts (CAFs) are key components of the hepatocellular carcinoma (HCC) tumor microenvironment (TME). regulating tumor proliferation, metastasis, therapy resistance, immune evasion via diverse mechanisms. A deeper understanding of the l diversity of CAFs is essential for predicting patient prognosis and guiding treatment strategies.
Methods: We examined the diversity of CAFs in HCC by integrating single-cell, bulk, and spatial transcriptome analyses.
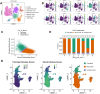
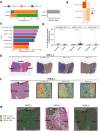
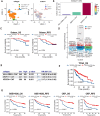
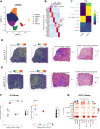
Results: Using a training cohort of 88 HCC single-cell RNA sequencing (scRNA-seq) samples and a validation cohort of 94 samples, encompassing over 1.2 million cells, we classified three fibroblast subpopulations in HCC: HLA-DRB1 + CAF, MMP11 + CAF, and VEGFA + CAF based on highly expressed genes of which, which are primarily located in normal tissue, tumor boundaries, and tumor interiors, respectively. Cell trajectory analysis revealed that VEGFA + CAFs are at the terminal stage of differentiation, which, notably, is tumor-specific. VEGFA + CAFs were significantly associated with patient survival, and the hypoxic microenvironment was found to be a major factor inducing VEGFA + CAFs. Through cellular communication with capillary endothelial cells (CapECs), VEGFA + CAFs promoted intra-tumoral angiogenesis, facilitating tumor progression and metastasis. Additionally, a machine learning model developed using high-expression genes from VEGFA + CAFs demonstrated high accuracy in predicting prognosis and sorafenib response in HCC patients.
Conclusions: We characterized three fibroblast subpopulations in HCC and revealed their distinct spatial distributions within the tumor. VEGFA + CAFs, which was induced by hypoxic TME, were associated with poorer prognosis, as they promote tumor angiogenesis through cellular communication with CapECs. Our findings provide novel insights and pave the way for individualized therapy in HCC patients.
Keywords: Angiogen esis; Hepatocellular carcinoma; Hypoxic microenvironment; Tumor-associated fibroblasts; VEGFA + CAF.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures


References
-
- Torimura T, Iwamoto H. Treatment and the prognosis of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2042–54. 10.1111/liv.15130. - PubMed
-
- Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO Guideline. J Clin Oncol. 2020;38:4317–45. 10.1200/jco.20.02672. - PubMed
-
- Song M, He J, Pan QZ, Yang J, Zhao J, Zhang YJ, Huang Y, Tang Y, Wang Q, He J, et al. Cancer-associated fibroblast-mediated cellular crosstalk supports hepatocellular carcinoma progression. Hepatology. 2021;73:1717–35. 10.1002/hep.31792. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

