Sex differences in the association of Alzheimer's disease biomarkers and cognition in a multicenter memory clinic study
- PMID: 39966925
- PMCID: PMC11837373
- DOI: 10.1186/s13195-025-01684-z
Sex differences in the association of Alzheimer's disease biomarkers and cognition in a multicenter memory clinic study
Abstract
Background: This study investigated sex differences in the associations between Alzheimer's disease (AD) biomarkers, cognitive performance, and decline in memory clinic settings.
Methods: 249 participants (females/males:123/126), who underwent tau-PET, amyloid-PET, structural MRI, and plasma glial fibrillary acidic protein (GFAP) measurement were included from Geneva and Lausanne Memory Clinics. Mann-Whitney U tests investigated sex differences in clinical and biomarker data. Linear regression models estimated the moderating effect of sex on the relationship between biomarkers and cognitive performance and decline. Sex differences in cognitive decline were further evaluated using longitudinal linear mixed-effect models with three-way interaction effects.
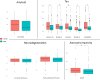
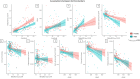
Results: Women and men present similar clinical features, amyloid, and neurodegeneration. Women had higher tau load and plasma levels of GFAP than men (p < 0.05). Tau associations with amyloid (standardized β = 0.54,p < 0.001), neurodegeneration (standardized β=-0.44,p < 0.001), and cognition (standardized β=-0.48,p < 0.001) were moderated by a significant interaction with sex. Specifically, the association between amyloid and tau was stronger among women than men (standardized β=-0.19,p = 0.047), whereas the associations between tau and cognition and between tau and neurodegeneration were stronger among men than in women (standardized β=-0.76,p = 0.001 and standardized β=-0.56,p = 0.044). Women exhibited faster cognitive decline than men in the presence of severe cortical thinning (p < 0.001).
Conclusion: Women showed higher tau load and stronger association between amyloid and tau than men. In individuals with high tau burden, men exhibited greater neurodegeneration and cognitive impairment than women. These findings support that sex differences may impact tau deposition through an upstream interplay with amyloid, leading to downstream effects on neurodegeneration and cognitive outcomes.
Keywords: Alzheimer’s disease; Biomarkers; Neuroimaging; Sex; Women; tau-PET.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Consent to participate: The local Ethics Committee approved the imaging studies, which have been conducted under the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice. Each subject or their relatives provided voluntary written informed consent to participate in the studies. Competing interests: VG received research support and speaker fees through her institution from Healthcare, Siemens Healthineers, Novo Nordisk. Janssen and Novartis. GBF has received support, payment, consulting fees, or honoraria through his institution for lectures, presentations, speaker bureaus, manuscript writing, or educations events from: Biogen, Roche, Diadem, Novo Nordisk, GE Healthcare, OM Pharma, and Eisai. GA has served at scientific advisory boards and consultants for Roche and Lilly and received honoraria for lectures from Lilly and Schwabe Pharma. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). KB has served as a consultant and at advisory boards for Acumen, ALZPath, BioArctic, Biogen, Eisai, Julius Clinical, Lilly, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials, and participated in educational programs for Biogen, Eisai, and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this article. The other authors have no conflicts of interest to disclose.
Figures

References
-
- Association A. Alzheimer’s disease facts and figures [published online ahead of print, 2020 Mar 10]. Alzheimers Dement. 2020. 2020.
-
- Hampel H, Cummings J, Blennow K, Gao P, Jack CR, Vergallo A. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat Rev Neurol [Internet]. 2021;17:580–9. Available from: 10.1038/s41582-021-00520-w - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

