GhMYB102 affects cotton fibre elongation and secondary wall thickening by regulating GhIRX10 in cotton
- PMID: 39966942
- PMCID: PMC11933854
- DOI: 10.1111/pbi.14588
GhMYB102 affects cotton fibre elongation and secondary wall thickening by regulating GhIRX10 in cotton
Abstract
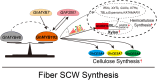
Upland cotton (Gossypium hirsutum) is a principal economic crop and a fundamental raw material for the textile industry. The quality of cotton fibres is significantly influenced by the synthesis of cell wall polysaccharides. This study focuses on GhIRX10, a beta-1,4-xylosyltransferase crucial for xylan backbone synthesis. Overexpression of GhIRX10 enhances xylan synthesis, which impacts fibre elongation and secondary cell wall thickening. GhMYB102, identified as a direct regulator of GhIRX10 expression, was confirmed through comprehensive validation. Overexpression of GhMYB102 resulted in a similar phenotype as OE-GhIRX10: increased cell wall thickness and reduced fibre length. Overexpression of GhMYB102 upregulated the expression of key cell wall synthesis-related genes, including GhCESA4/7/8, GhIRXs, GhCESAs, GhGUXs, GhTBLs, GhXTHs, and GhXXTs. Consequently, the cellulose and hemicellulose contents in OE-GhMYB102 lines were significantly increased. GhMYB102 was also validated as a target gene regulated by GhFSN1 and GhMYB7, with the ability to reciprocally regulate GhFSN1 expression. In summary, we propose a regulatory model where GhMYB102 promotes the expression of GhIRX10 and other cell wall-related genes, thereby affecting fibre quality. This study elucidates the regulatory network of secondary cell wall synthesis in cotton and provides potential targets for improving fibre quality through molecular breeding.
Keywords: GhIRX10; GhMYB102; cotton; fibre development; secondary cell wall; xylan.
© 2025 The Author(s). Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Brown, D.M. , Zhang, Z. , Stephens, E. , Dupree, P. and Turner, S.R. (2009) Characterization of IRX10 and IRX10‐like reveals an essential role in glucuronoxylan biosynthesis in Arabidopsis . Plant J. 57, 732–746. - PubMed
-
- Chen, X. , Vega‐Sánchez, M.E. , Verhertbruggen, Y. , Chiniquy, D. , Canlas, P.E. , Fagerström, A. , Prak, L. et al. (2013) Inactivation of OsIRX10 leads to decreased Xylan content in rice culm cell walls and improved biomass saccharification. Mol. Plant 6, 570–573. - PubMed
MeSH terms
Substances
Grants and funding
- Agricultural Science and Technology Innovation Program of Chinese Academy of Agricultural Sciences
- 2022D01B222/Natural Science Foundation of Xinjiang Uygur Autonomous Region
- 2023ZD04041/China Project of Cotton Biological Breeding
- 242300421116/Natural Science Foundation of Henan Province
- 202203021222416/Natural Science Foundation of Shanxi Province
LinkOut - more resources
Full Text Sources

