Cardiovascular Safety in Oncology Clinical Trials: JACC: CardioOncology Primer
- PMID: 39967209
- PMCID: PMC11866450
- DOI: 10.1016/j.jaccao.2024.09.014
Cardiovascular Safety in Oncology Clinical Trials: JACC: CardioOncology Primer
Abstract
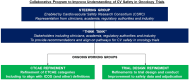
The development of novel treatments has improved cancer outcomes but may result in cardiovascular toxicities. Traditional approaches to clinical trial safety evaluation have limitations in their ability to detect signals of cardiovascular risk. Mechanisms to increase power and specificity to clarify cardiovascular safety are required. However, implications include increased costs and slower development. The Cardiovascular Safety Research Consortium facilitated stakeholder discussions with representation from academia, industry, and regulators. A think tank was assembled with the aim of providing recommendations for improved collection and reporting of cardiovascular safety signals in oncology trials. Two working groups were formed. The first focuses on incorporation of consensus definitions of cardiovascular disease into the Common Terminology Criteria for Adverse Events used in oncology trial reporting. The second group considers methods for ascertainment and adjudication of cardiovascular events in cancer trials. The overarching aim of this primer is to improve understanding of the potential cardiovascular toxicities of cancer therapies.
Keywords: adverse events; cardiotoxicity; chemotherapy; heart failure; immunotherapy; outcomes; reporting; targeted therapy.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Funding Support and Author Disclosures Dr Bonaca has received support from the American Heart Association SFRN under award numbers 18SFRN3390085 (BWH-DH SFRN Center) and 18SFRN33960262 (BWH-DH Clinical Project). Dr Lang is supported by British Heart Foundation Centre of Research Excellence Award RE/18/6/34217. Dr Deswal is supported in part by the Ting Tsung and Wei Fong Chao Distinguished Chair. Dr Moslehi is supported by National Institutes of Health grants R01HL141466, R01HL155990, R01HL156021, R01HL160688, and R01HL170038. Dr Bonaca is the executive director of CPC Clinical Research, a nonprofit academic research organization affiliated with the University of Colorado; has received research grant/consulting funding between August 2021 and present from: Abbott Laboratories, Agios Pharmaceuticals, Inc, Alexion Pharma, Alnylam Pharmaceuticals, Inc, Amgen Inc, Angionetics, Inc, Anthos Therapeutics, ARCA Biopharma, Inc, Array BioPharma, Inc, AstraZeneca and Affiliates, Atentiv LLC, Audentes Therapeutics, Inc, Bayer and Affiliates, Beth Israel Deaconess Medical Center, Better Therapeutics, Inc, Boston Clinical Research Institute, Bristol-Meyers Squibb Company, Cambrian Biopharma, Inc, Cardiol Therapeutics Inc, CellResearch Corp, Cleerly Inc, Cook Regentec LLC, CSL Behring LLC, Eidos Therapeutics, Inc, EP Trading Co Ltd, EPG Communication Holdings Ltd, Epizon Pharma, Inc, Esperion Therapeutics, Inc, Everly Well, Inc, Exicon Consulting Pvt Ltd, Faraday Pharmaceuticals, Inc, Foresee Pharmaceuticals Co Ltd, Fortress Biotech, Inc, HDL Therapeutics Inc, HeartFlow Inc, Hummingbird Bioscience, Insmed Inc, Ionis Pharmaceuticals, IQVIA Inc, Janssen and Affiliates, Kowa Research Institute, Inc, Kyushu University, Lexicon Pharmaceuticals, Inc, Medimmune Ltd, Medpace, Merck & Affiliates, Nectero Medical Inc, Novartis Pharmaceuticals Corp, Novo Nordisk, Inc, Osiris Therapeutics Inc, Pfizer Inc, PhaseBio Pharmaceuticals, Inc, PPD Development, LP, Prairie Education and Research Cooperative, Prothena Biosciences Limited, Regeneron Pharmaceuticals, Inc, Regio Biosciences, Inc, Saint Luke’s Hospital of Kansas City, Sanifit Therapeutics S.A., Sanofi-Aventis Groupe, Silence Therapeutics PLC, Silence, Smith & Nephew plc, Stanford Center for Clinical Research, Stealth BioTherapeutics Inc, State of Colorado CCPD Grant, The Brigham & Women's Hospital, Inc, The Feinstein Institutes for Medical Research, Thrombosis Research Institute, University of Colorado, University of Pittsburgh, VarmX, Virta Health Corporation, Worldwide Clinical Trials Inc, WraSer, LLC, and Yale Cardiovascular Research Group. Dr Lang has received research grants from Roche Diagnostics, AstraZeneca, and Boehringer Ingelheim; and consultancy/speaker fees from Roche Diagnostics, Myokardia, Pharmacosmos, Akero Therapeutics, CV6 Therapeutics, Jazz Pharma, and Novartis outside the submitted work. Dr Chen reports that the National Cancer Institute has Cooperative Research and Development Agreements with Genentech and AstraZeneca for trials in which she is the principal investigator. Dr Lipka is an employee of Merck & Co; and owns stock in Merck & Co. Dr Janicijevic is employee of F. Hoffmann-La Roche Ltd, Basel, Switzerland; and has nonvoting shares in F. Hoffmann-La Roche. Dr Herrmann has served on advisory boards for AstraZeneca, Astellas, and Pfizer. Dr de Boer has received research grants and/or fees from AstraZeneca, Abbott, Boehringer Ingelheim, Cardior Pharmaceuticals GmbH, Novo Nordisk, and Roche; and has had speaker engagements with and/or received fees from Abbott, AstraZeneca, Bristol Myers Squibb, Cardior Pharmaceuticals GmbH, Novartis, and Roche. Dr Deswal has received research support from the NIH and CPRIT; and consultancy fees from Bayer. Dr Ky is the editor-in-chief of JACC: CardioOncology; has received research funding from Pfizer; has received honoraria from UpToDate and Medscape; and has been a consultant to AstraZeneca. Dr Dent has been a consultant to AstraZeneca, Novartis, Pfizer, Race Oncology, Myocardial Solutions, Bristol Myers Squibb, and Gilead Sciences. Dr Fernandez has received honoraria or consultation fees from Philips, Myocardial Solutions, Bayer, Daiichi Sankyo, AstraZeneca, Beigene, Janssen, and Pfizer outside the submitted work. Dr Cornell is employed at AbbVie; and owns stock in AbbVie. Dr Flaig has a leadership role and owns stock in Aurora Oncology; has been a consultant to Seagen and Janssen Oncology; has received research support from Novartis, Bavarian Nordic, Dendreon, GTx, Janssen Oncology, Medivation, Sanofi, Pfizer, Bristol Myers Squibb, Roche/Genentech, Exelixis, Aragon Pharmaceuticals, Sotio, Tokai Pharmaceuticals, AstraZeneca/MedImmune, Eli Lilly, Astellas Pharma, Agensys, Seagen, La Roche-Posay, Merck, Myovant Sciences, and Criterium; and is the inventor on patents filed by the University of Colorado. Dr de Azambuja has received honoraria from and/or served on advisory boards for Roche/GNE, Novartis, SeaGen, Zodiac, Libbs, Pierre Fabre, Eli Lilly, AstraZeneca, MSD, and Gilead Sciences; has received travel grants from Roche/GNE and AstraZeneca; has received institutional research grants from Roche/GNE, AstraZeneca, GSK/Novartis, and Gilead Sciences. Dr Seltzer has served on safety committee for AstraZeneca, Minoryx, Moderna, Vivoryon Therapeutics, Pathalys Pharma, Takeda, and Crinetics. Dr Neilan has been a consultant to and received fees from Parexel Imaging, Bristol Myers Squibb, Roivant, Intrinsic Imaging, H3-Biomedicine, Amgen, Sanofi, Genentech, Roche, and AbbVie; and has received research grant funding from Bristol Myers Squibb, Abbott, and AstraZeneca. Dr Moleshi has served on advisory boards for Bristol Myers Squibb, Takeda, AstraZeneca, Myovant, Kurome Therapeutics, Kiniksa Pharmaceuticals, Daiichi Sankyo, CRC Oncology, BeiGene, Prelude Therapeutics, TransThera Sciences, and Cytokinetics. Dr Januzzi is a trustee of the American College of Cardiology; is a director at Imbria Pharmaceuticals; is an advisor at Jana Care; has received grant support from Abbott, Applied Therapeutics, AstraZeneca, HeartFlow Inc, Innolife, and Roche Diagnostics; has received consulting income from Abbott, Beckman-Coulter, Boehringer Ingelheim, Janssen, Novartis, Merck, Roche Diagnostics, Siemens, Quidel-Ortho; and participates in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Bayer, CVRx, Intercept, Pfizer, and Takeda. Dr Petrie has received research funding from Boehringer Ingelheim, Roche, SQ Innovations, AstraZeneca, Novartis, Novo Nordisk, Medtronic, Boston Scientific, and Pharmacosmos; and has been a consultant and/or served on trial committees for Akero, Applied Therapeutics, AnaCardio, Biosensors, Boehringer Ingelheim, Novartis, AstraZeneca, Novo Nordisk, AbbVie, Bayer, Takeda, Cardiorentis, Pharmacosmos, Siemens, Eli Lilly, Vifor, New Amsterdam, Moderna, AnaCardio, and Teikuko. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures





References
-
- Moslehi J.J. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457–1467. - PubMed
-
- Addison D., Branch M., Baik A.H., et al. Equity in cardio-oncology care and research: a scientific statement from the American Heart Association. Circulation. 2023;148:297–308. - PubMed

