Individualized Net Benefit of Intensive Blood Pressure Lowering Among Community-Dwelling Older Adults in SPRINT
- PMID: 39967308
- PMCID: PMC12100678
- DOI: 10.1111/jgs.19395
Individualized Net Benefit of Intensive Blood Pressure Lowering Among Community-Dwelling Older Adults in SPRINT
Abstract
Background: The optimal blood pressure (BP) target for older adults with hypertension remains controversial, particularly among those with advanced age, frailty, or polypharmacy. This study estimated the individualized net benefit of intensive BP lowering among community-dwelling older adults in the Systolic Blood Pressure Intervention Trial (SPRINT).
Methods: Among 5143 SPRINT participants age ≥ 65 years, Cox models were internally validated to predict an absolute difference in risk between treating to a systolic BP target of < 120 versus < 140 mm Hg for all-cause death, cardiovascular outcomes, cognitive outcomes, and serious adverse events. Treatment effects were combined using simulated preference weights into individualized net benefits, representing the weighted sum of risk differences across outcomes. Net benefits were compared across categories of age (65-74 vs. ≥ 75 years), SPRINT-derived frailty status (fit, less fit, and frail), and polypharmacy (≥ 5 medications).
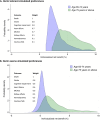
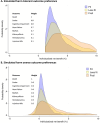
Results: When simulating preferences for participants who view the benefits of BP lowering (reduction in death, cardiovascular events, and cognitive impairment) as much more important than treatment-related harms (e.g., acute kidney injury and syncope), the median net benefit from intensive BP lowering was 4 percentage points (IQR: 3-6), and 100% had a positive net benefit favoring intensive BP lowering. When simulating benefits and harms to have similar, intermediate importance, the median net benefit was 1 percentage point (IQR: 0-2), and 85% had a positive net benefit. Participants with advanced age and frailty had greater net benefits from intensive BP lowering despite experiencing more harm in both simulations, and those with polypharmacy had greater net benefits when benefits were viewed as much more important than harms (p < 0.001 for all comparisons).
Conclusions: Among community-dwelling older adults with hypertension in SPRINT, almost all participants had a net benefit that favored a systolic BP target of < 120 mm Hg, but the magnitude of net benefit varied according to estimated risks and simulated preferences.
Keywords: frailty; hypertension; older adults; polypharmacy; shared decision‐making.
© 2025 The Author(s). Journal of the American Geriatrics Society published by Wiley Periodicals LLC on behalf of The American Geriatrics Society.
Conflict of interest statement
M.M.E. has served on advisory expert panels for Boehringer Ingelheim Inc. and has a research collaborative agreement with Bayer Inc. M.G.S. has received consulting income from Cricket Health Inc.; has served on advisory panels for Boehringer Ingelheim, Astra Zeneca, and Bayer; and has received research support from Bayer. J.H.I. holds an investigator‐initiated research grant from Baxter International Inc., serves as a member of a data safety monitoring board for Sanifit Therapeutics, is a member of the scientific advisory board for Alpha Young, and has served on advisory boards for AstraZeneca and Ardelyx. J.D.B. reports grant support from the NIH, Roche Diagnostics, and Abbott Diagnostics, consulting fees from Roche Diagnostics, Astra Zeneca, and the Cooper Institute. J.A.d.L. reports grant support from Roche Diagnostics and Abbott Diagnostics, consulting fees from Roche Diagnostics, Abbott Diagnostics, Ortho Clinical Diagnostics, Beckman Coulter, Quidel Cardiovascular Inc., and Siemen's Health Care Diagnostics. He has been named a co‐owner on a patent awarded to the University of Maryland (U.S. Patent Application Number: 15/309,754) entitled: “Methods for Assessing Differential Risk for Developing Heart Failure.” The remaining authors have nothing to disclose.
Figures

References
-
- Collaborators GBDRF , “Global, Regional, and National Comparative Risk Assessment of 79 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks, 1990‐2015: A Systematic Analysis for the Global Burden of Disease Study 2015,” Lancet 388, no. 10053 (2016): 1659–1724, 10.1016/S0140-6736(16)31679-8. - DOI - PMC - PubMed
-
- Blood Pressure Lowering Treatment Trialists C , “Pharmacological Blood Pressure Lowering for Primary and Secondary Prevention of Cardiovascular Disease Across Different Levels of Blood Pressure: An Individual Participant‐Level Data Meta‐Analysis,” Lancet 397, no. 10285 (2021): 1625–1636, 10.1016/S0140-6736(21)00590-0. - DOI - PMC - PubMed
-
- Bress A. P., Kramer H., Khatib R., et al., “Potential Deaths Averted and Serious Adverse Events Incurred From Adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) Intensive Blood Pressure Regimen in the United States: Projections From NHANES (National Health and Nutrition Examination Survey),” Circulation 135, no. 17 (2017): 1617–1628, 10.1161/CIRCULATIONAHA.116.025322. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1K24HL166681-01/HL/NHLBI NIH HHS/United States
- 5R01DK098234-08/DK/NIDDK NIH HHS/United States
- K24DK110427/DK/NIDDK NIH HHS/United States
- 14EIA18560026/American Heart Association
- 936281/American Heart Association
- American Society of Nephrology, KidneyCure Ben J. Lipps Research Grant
- KL2 TR001859/TR/NCATS NIH HHS/United States
- UL1 TR001860/TR/NCATS NIH HHS/United States
- 1K24HL166681-01/HL/NHLBI NIH HHS/United States
- 5R01DK098234-08/DK/NIDDK NIH HHS/United States
- K24DK110427/DK/NIDDK NIH HHS/United States
- KL2 TR001859/TR/NCATS NIH HHS/United States
- UL1 TR001860/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

