Material surface conjugated with fibroblast growth factor-2 for pluripotent stem cell culture and differentiation
- PMID: 39967781
- PMCID: PMC11835233
- DOI: 10.1093/rb/rbaf003
Material surface conjugated with fibroblast growth factor-2 for pluripotent stem cell culture and differentiation
Abstract
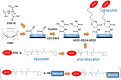
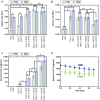
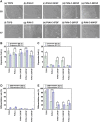
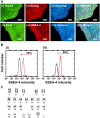
Fibroblast growth factor-2 (FGF-2) is a critical molecule for sustaining the pluripotency of human pluripotent stem (PS) cells. However, FGF-2 is extremely unstable and cannot be stored long periods at room temperature. Therefore, the following FGF-2-conjugated cell culture materials were developed to stabilize FGF-2: FGF-2-conjugated polyvinyl alcohol (PVAI-C-FGF) hydrogels and FGF-2-conjugated carboxymethyl cellulose-coated (CMC-C-FGF) dishes. Human induced pluripotent stem (iPS) cells were proliferated on recombinant vitronectin (rVN)-coated PVAI-C-FGF hydrogels and CMC-C-FGF dishes in medium without FGF-2. Human iPS cells could not be cultivated on rVN-coated PVAI-C-FGF hydrogels for more than two passages but could proliferate on rVN-coated CMC-C-FGF dishes. These results indicated that the amount of immobilized FGF-2 and the base cell materials are important, including the amount of immobilized rVN and the conformation of FGF-2 on the surfaces. When human iPS cells were proliferated on rVN-coated CMC-C-FGF surfaces in medium containing no FGF-2 for 10 passages, their pluripotency and potential to differentiate into cells originating from three germ layers were maintained in vivo and in vitro. Furthermore, the cells could extensively differentiate into cardiomyocytes, which can be used for cardiac infarction treatment in future and retinal pigment epithelium for retinal pigmentosa treatment in future. The FGF-2-immobilized surface could enable human PS cell culture in medium that does not need to contain unstable FGF-2. The amount of FGF-2 immobilization on the rVN-coated CMC-C-5FGF and CMC-C-20FGF dishes was reduced to 93.6 and 52.2 times, respectively, which is less than the conventional amount of FGF-2 used in culture medium for one passage (6 days) of human iPS cell culture. This reduction resulted from the stabilization of unstable FGF-2 by the immobilization of FGF-2, which was achieved by utilizing optimal base materials (CMC), coating materials (rVN) and long-joint segment (PEG4-SPDP) design.
Keywords: carboxymethyl cellulose; cell differentiation; fibroblast growth factor-2; human pluripotent stem cells; material design.
© The Author(s) 2025. Published by Oxford University Press.
Figures




References
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–72. - PubMed
-
- Higuchi A, Kumar SS, Benelli G, Ling QD, Li HF, Alarfaj AA, Munusamy MA, Sung TC, Chang Y, Murugan K. Biomaterials used in stem cell therapy for spinal cord injury. Prog Mater Sci 2019;103:374–424.
LinkOut - more resources
Full Text Sources
Research Materials

