Competing endogenous RNA networks in ovarian cancer: from bench to bedside
- PMID: 39967908
- PMCID: PMC11830916
- DOI: 10.17179/excli2024-7827
Competing endogenous RNA networks in ovarian cancer: from bench to bedside
Abstract
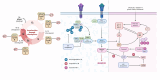
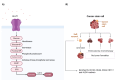
Epithelial ovarian cancer is responsible for the majority of ovarian malignancies, and its highly invasive nature and chemoresistant development have been major obstacles to treating patients with mainstream treatments. In recent decades, the significance of microRNAs (miRNAs), circular RNAs (circRNAs), long non-coding RNAs (lncRNAs), and competing endogenous RNAs (ceRNAs) has been highlighted in ovarian cancer development. This hidden language between these RNAs has led to the discovery of enormous regulatory networks in ovarian cancer cells that substantially affect gene expression. Aside from providing ample opportunities for targeted therapies, circRNA- and lncRNA-mediated ceRNA network components provide invaluable biomarkers. The current study provides a comprehensive and up-to-date review of the recent findings on the significance of these ceRNA networks in the hallmarks of ovarian cancer oncogenesis, treatment, diagnosis, and prognosis. Also, it provides the authorship with future perspectives in the era of single-cell RNA sequencing and personalized medicine.
Keywords: circular RNA; competing endogenous RNAs; long non-coding RNA; microRNAs; ovarian cancer.
Copyright © 2025 Derogar et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Abdoli Shadbad M, Hemmat N, Khaze Shahgoli V, Derakhshani A, Baradaran F, Brunetti O, et al. A systematic review on pd-1 blockade and pd-1 gene-editing of CAR-T cells for glioma therapy: from deciphering to personalized medicine. Front Immunol. 2021;12:788211. doi: 10.3389/fimmu.2021.788211. Available from: http://dx.doi.org/10.3389/fimmu.2021.788211. - DOI - PMC - PubMed
-
- Aichen Z, Kun W, Xiaochun S, Lingling T. LncRNA FGD5-AS1 promotes the malignant phenotypes of ovarian cancer cells via targeting miR-142-5p. Apoptosis. 2021;26:348–360. doi: 10.1007/s10495-021-01674-0. Available from: http://dx.doi.org/10.1007/s10495-021-01674-0. - DOI - PubMed
-
- Alessio E, Bonadio RS, Buson L, Chemello F, Cagnin S. A single cell but many different transcripts: a journey into the world of long non-coding RNAs. Int J Mol Sci. 2020;21(1):302. doi: 10.3390/ijms21010302. Available from: http://dx.doi.org/10.3390/ijms21010302. - DOI - PMC - PubMed
-
- An J, Lv W, Zhang Y. LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. Onco Targets Ther. 2017;10:5377–5390. doi: 10.2147/ott.s147586. Available from: http://dx.doi.org/10.2147/ott.s147586. - DOI - PMC - PubMed
-
- Arora T, Mullangi S, Lekkala MR. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2021. Ovarian cancer.
Publication types
LinkOut - more resources
Full Text Sources
