Clinical and Economic Evaluation of Fluticasone Furoate/Umeclidinium/Vilanterol Versus Tiotropium/Olodaterol Therapy in Maintenance Treatment-Naive Patients with COPD in the US
- PMID: 39968202
- PMCID: PMC11834697
- DOI: 10.2147/COPD.S479504
Clinical and Economic Evaluation of Fluticasone Furoate/Umeclidinium/Vilanterol Versus Tiotropium/Olodaterol Therapy in Maintenance Treatment-Naive Patients with COPD in the US
Abstract
Purpose: Long-acting bronchodilator (LABD) therapy is recommended for maintenance treatment in most patients with chronic obstructive pulmonary disease (COPD). However, triple therapy (TT; dual LABDs + inhaled corticosteroid [ICS]) is often used as first-line maintenance treatment. The benefits of TT versus dual LABDs as first-line treatments are unknown, necessitating an evaluation of its effectiveness and costs versus non-ICS alternatives.
Patients and methods: This retrospective study assessed administrative claims of maintenance treatment-naive patients in the United States with COPD aged ≥40 years initiating single-inhaler fluticasone furoate+umeclidinium+vilanterol (FF+UMEC+VI) or tiotropium+olodaterol (TIO+OLO). Patients were propensity score-matched (1:1) and followed for up to 12 months. The primary outcome was time to first COPD exacerbation. Secondary outcomes included time to first pneumonia diagnosis, pneumonia-related hospitalization, healthcare resource utilization (HCRU), and costs. COPD exacerbation and pneumonia risk were assessed using Cox proportional hazards regression.
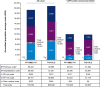
Results: A total of 5,121 and 3,996 patients met the eligibility criteria for the FF+UMEC+VI and TIO+OLO groups, respectively. Outcomes were assessed among 2,951 matched pairs. The risk of moderate or severe COPD exacerbation was not significantly different between FF+UMEC+VI and TIO+OLO groups (hazard ratio [HR] [95% confidence interval {CI}]: 1.13 [0.99-1.29]; P=0.064). The risks of pneumonia (HR [95% CI]: 1.04 [0.85-1.27]; P=0.723) and pneumonia-related hospitalization (HR [95% CI]: 1.18 [0.78-1.79]; P=0.429) were also not significantly different between the groups. There were no significant differences in HCRU events or all-cause costs; however, FF+UMEC+VI initiators incurred greater COPD- and/or pneumonia-related pharmacy costs than TIO+OLO initiators (FF+UMEC+VI: $2,934 [$2,827-$3,041], TIO+OLO: $1,994 [$1,915-$2,073]; P<0.001).
Conclusion: In maintenance treatment-naive patients, FF+UMEC+VI offered no reduction in COPD exacerbation risk over TIO+OLO and resulted in higher pharmacy costs related to COPD and/or pneumonia treatment. These results support treatment recommendations for LAMA+LABA as initial maintenance therapy.
Trial registration: ClinicalTrials.gov identifier - NCT05169424.
Keywords: dual bronchodilator therapy; exacerbation risk; health outcomes; maintenance treatment–naive; treatment initiation; triple therapy.
Plain language summary
Chronic obstructive pulmonary disease (COPD) is a disease affecting the lungs, which causes symptoms such as shortness of breath, cough, and phlegm. The goal of COPD management is to control the symptoms and reduce the risk of flare-ups (exacerbations). COPD maintenance treatments include medications called inhaled corticosteroids (ICS) that reduce airway inflammation and bronchodilators that either prevent the closing of airways (eg, long-acting muscarinic antagonists [LAMAs]), or keep them open longer (eg, long-acting beta2-agonists [LABAs]). National and international guidelines recommend triple therapy (ICS+LAMA+LABA) for symptomatic patients who continue to have frequent exacerbations despite LAMA+LABA dual therapy. However, the use of triple therapy as the first treatment choice is common in everyday clinical practice, even in patients who have not received any long-acting bronchodilators in the past (maintenance treatment–naive patients). Therefore, our study compared the clinical and economic outcomes in maintenance treatment–naive patients who were given single-inhaler triple therapy (LAMA+LABA+ICS) with those who were given dual therapy (LAMA+LABA). In our study, the risk of COPD flare-up, pneumonia, and hospitalization due to pneumonia was not different between patients who received triple and dual therapy, indicating no significant benefit of triple therapy over dual therapy. Additionally, triple therapy resulted in higher pharmacy costs. We conclude that for patients with COPD starting maintenance treatment, dual therapy was not only as effective as triple therapy in managing COPD but also had economic benefits.
© 2025 Shaikh et al.
Conflict of interest statement
Brendan Clark is an employee of BIPI, and Asif Shaikh and Swetha R. Palli were employees of BIPI at the time of the study. At the time the study was conducted, John Ritz was an employee of Syneos Health and was contracted and compensated by BIPI. Julian Casciano and Zenobia Dotiwala are employees of eMax Health. The authors received no direct compensation for the development of this manuscript. Jennifer K. Quint received grants from the Health Foundation, MRC, GlaxoSmithKline, Bayer, BIPI, Asthma UK/British Lung Foundation, Health Data Research UK, Chiesi, Sanofi, and AstraZeneca and personal fees for advisory board participation or speaking fees from GlaxoSmithKline, BIPI, AstraZeneca, Chiesi, Insmed, and Bayer outside the submitted work. The authors report no other conflicts of interest in this work.
Figures

References
-
- Bracke KR, Brusselle GG. Chapter 97 - chronic obstructive pulmonary disease. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroutre H, Lambrecht BN, editors. Mucosal Immunology. 4th ed. Boston: Academic Press; 2015:1857–1866.
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). The global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2024. Available from: https://goldcopd.org/2024-gold-report/. Accessed December 28, 2023.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

