Histidine triad nucleotide-binding protein 2 attenuates doxorubicin-induced cardiotoxicity through restoring lysosomal function and promoting autophagy in mice
- PMID: 39968501
- PMCID: PMC11831189
- DOI: 10.1002/mco2.70075
Histidine triad nucleotide-binding protein 2 attenuates doxorubicin-induced cardiotoxicity through restoring lysosomal function and promoting autophagy in mice
Abstract
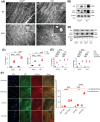
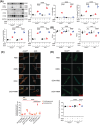
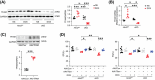
Doxorubicin (DOX) is an effective chemotherapy drug widely used against various cancers but is limited by severe cardiotoxicity. Mitochondria-lysosome interactions are crucial for cellular homeostasis. This study investigates the role of histidine triad nucleotide-binding protein 2 (HINT2) in DOX-induced cardiotoxicity (DIC). We found that HINT2 expression was significantly upregulated in the hearts of DOX-treated mice. Cardiac-specific Hint2 knockout mice exhibited significantly worse cardiac dysfunction, impaired autophagic flux, and lysosomal dysfunction after DOX treatment. Mechanistically, HINT2 deficiency reduced oxidative phosphorylation complex I activity and disrupted the nicotinamide adenine dinucleotide NAD+/NADH ratio, impairing lysosomal function. Further, HINT2 deficiency suppressed sterol regulatory element binding protein 2 activity, downregulating transcription factor A mitochondrial, a critical regulator of complex I. Nicotinamide mononucleotide (NMN) supplementation restored lysosomal function in vitro, while cardiac-specific Hint2 overexpression using adeno-associated virus 9 or adenovirus alleviated DIC both in vivo and in vitro. These findings highlight HINT2 as a key cardioprotective factor that mitigates DIC by restoring the NAD+/NADH ratio, lysosomal function, and autophagy. Therapeutic strategies enhancing HINT2 expression or supplementing NMN may reduce cardiac damage and heart failure caused by DOX.
Keywords: HINT2; NAD+/NADH; autophagy; doxorubicin; lysosome.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Barry E, Alvarez JA, Scully RE, Miller TL, Lipshultz SE. Anthracycline‐induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin Pharmacother. 2007;8(8):1039‐1058. - PubMed
-
- Vasquez‐Vivar J, Martasek P, Hogg N, Masters BS, Pritchard KA, Jr , Kalyanaraman B. Endothelial nitric oxide synthase‐dependent superoxide generation from adriamycin. Biochemistry. 1997;36(38):11293‐11297. - PubMed
-
- Berthiaume JM, Wallace KB. Adriamycin‐induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol. 2007;23(1):15‐25. - PubMed
-
- Hasinoff BB, Schnabl KL, Marusak RA, Patel D, Huebner E. Dexrazoxane (ICRF‐187) protects cardiac myocytes against doxorubicin by preventing damage to mitochondria. Cardiovasc Toxicol. 2003;3(2):89‐99. - PubMed
LinkOut - more resources
Full Text Sources
