A synthetic platform for developing recombinant adeno-associated virus type 8 producer cell lines
- PMID: 39968660
- PMCID: PMC12171320
- DOI: 10.1002/btpr.70009
A synthetic platform for developing recombinant adeno-associated virus type 8 producer cell lines
Abstract
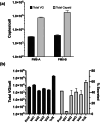
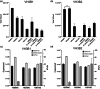
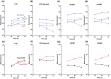
Recombinant adeno-associated virus (rAAV) is one of the most widely used viral vectors for gene therapy. It is used in very high doses for the treatment of many diseases, making large-scale production for clinical applications challenging. We have established a synthetic biology-based platform to construct stable production cell lines, which can be induced to produce rAAV2. In this study, we extended our cell line construction pipelines for rAAV2 to rAAV8, a serotype whose tropism makes it attractive for gene delivery in multiple tissues. The Genome Module, encoding the rAAV2 genome, and Replication Modules, containing Rep68, DBP and E4orf6 coding sequences, originally used for rAAV2 were retained, but the Packaging Module was modified to replace the AAV2 intron-less cap gene (VP123) with that of AAV8. These three genetic modules were integrated into HEK293 genome to generate four rAAV8 producer cell lines VH1-4, which all produced rAAV8 upon induction. Their productivity was similar to the initial rAAV2 producer cell lines GX2/6 constructed using the same pipeline, but was much lower than conventional triple plasmid transfection. We identified Cap protein production and capsid formation as a potential limiting factor, just as we observed in GX2/6. By integrating more copies of AAV8 VP123 into VH3 clone, the encapsidated rAAV8 titer increased 20-fold to a level comparable to triple transfection. By tuning induction conditions to modulate capsid production, the full particle content could be elevated. This study demonstrated that our rAAV producer cell line development platform is robust and applicable to different AAV serotypes.
Keywords: HEK293; adeno‐associated virus; biomanufacturing; gene therapy; synthetic biology.
© 2025 The Author(s). Biotechnology Progress published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

