Cardiovascular Outcomes With Icosapent Ethyl by Baseline Low-Density Lipoprotein Cholesterol: A Secondary Analysis of the REDUCE-IT Randomized Trial
- PMID: 39968782
- PMCID: PMC12132757
- DOI: 10.1161/JAHA.124.038656
Cardiovascular Outcomes With Icosapent Ethyl by Baseline Low-Density Lipoprotein Cholesterol: A Secondary Analysis of the REDUCE-IT Randomized Trial
Abstract
Background: The efficacy of icosapent ethyl among patients with very well-controlled baseline low-density lipoprotein cholesterol (LDL-C) is unknown.
Methods: In this post hoc analysis of the REDUCE-IT (Reduction of Cardiovascular Events With Icosapent Ethyl-Intervention Trial) randomized clinical trial, statin-treated patients with high cardiovascular risk, elevated triglycerides (135-499 mg/dL), and baseline LDL-C of 41 to 100 mg/dL were included. Patients were randomized to icosapent ethyl (2 g twice daily) or placebo and then post hoc stratified by baseline LDL-C (<55 mg/dL versus ≥55 mg/dL). The primary composite end point included cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina.
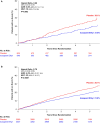
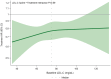
Results: Among 8175 patients with baseline LDL-C data, 7117 (87.1%) had LDL-C ≥55 mg/dL and 1058 (12.9%) had LDL-C <55 mg/dL. In patients with LDL-C <55 mg/dL, the rate of the primary composite end point was lower in the icosapent ethyl group (16.2% versus 22.8%) than in the placebo group (hazard ratio [HR], 0.66 [95% CI, 0.50-0.87]; absolute risk reduction, 6.6%; P=0.003). Among patients with LDL-C ≥55 mg/dL, a primary composite end point event occurred in a lower proportion of patients in the icosapent ethyl group (17.4% versus 21.9%) than in the placebo group (HR, 0.76 [95% CI, 0.69-0.85]; absolute risk reduction, 4.5%; P<0.0001). No significant interaction was observed between baseline LDL-C and treatment group (P for interaction=0.40). Findings were consistent among secondary cardiovascular end points and in sensitivity analyses.
Conclusions: Among statin-treated patients with elevated triglycerides and high cardiovascular risk, icosapent ethyl reduced the rate of cardiovascular end points irrespective of baseline LDL-C, including among eligible patients with optimal LDL-C control.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT01492361.
Keywords: cardiovascular outcomes; icosapent ethyl; low‐density lipoprotein cholesterol.
Figures


References
-
- Klempfner R, Erez A, Sagit B‐Z, Goldenberg I, Fisman E, Kopel E, Shlomo N, Israel A, Tenenbaum A. Elevated triglyceride level is independently associated with increased all‐cause mortality in patients with established coronary heart disease: twenty‐two‐year follow‐up of the bezafibrate infarction prevention study and registry. Circ Cardiovasc Qual Outcomes. 2016;9:100–108. doi: 10.1161/CIRCOUTCOMES.115.002104 - DOI - PubMed
-
- Bays HE, Ballantyne CM, Braeckman RA, Stirtan WG, Soni PN. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs Drugs Devices Interv. 2013;13:37–46. doi: 10.1007/s40256-012-0002-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

