Systolic and diastolic dysfunction is exacerbated by age and spinal cord injury in male and female mice with central nervous system serotonin deficiency
- PMID: 39968856
- PMCID: PMC11908478
- DOI: 10.1113/JP287067
Systolic and diastolic dysfunction is exacerbated by age and spinal cord injury in male and female mice with central nervous system serotonin deficiency
Abstract
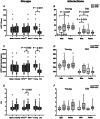
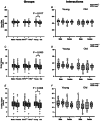
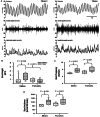
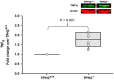
The present study was designed to explore whether the depletion of serotonin (5-HT) in the central nervous system (CNS5-HT) leads to systolic and diastolic dysfunction and whether this dysfunction is exacerbated by sex, age and spinal cord injury. Echocardiographic assessment of systolic and diastolic function was completed in young and old male and female tryptophan hydroxylase 2 knockout (TPH2-/-) and wild-type (TPH2+/+) mice with intact spinal cords, as well as in C2 spinal cord hemisected young TPH2-/- and TPH2+/+ mice. In addition, lumbar sympathetic nervous system activity was recorded in elderly male and female intact TPH2-/- and TPH2+/+ mice. Systolic and diastolic dysfunction was evident in young TPH2-/- mice, including a higher left ventricular mass (P < 0.001), left ventricular outflow parameters (e.g. peak velocity) and E/A (P < 0.001). Reductions in ejection fraction and fractional shortening were also evident (P < 0.001), although stroke volume and cardiac output were maintained. The assessed dysfunction was exacerbated by age and spinal cord injury, resulting in reductions in cardiac output (P ≤ 0.01). The dysfunction was accompanied by increases in sympathetic burst height (P = 0.038) and incidence (P = 0.001). Reductions in CNS5-HT are coupled to systolic and diastolic dysfunction, which is exacerbated by age and spinal cord injury. This dysfunction is coupled to increases in sympathetic nervous system activity in elderly mice. Our findings are an initial step toward determining whether reductions in CNS5-HT are a unifying mechanism that links central sleep apnoea, sympathoexcitation and heart failure in intact and spinal cord injured individuals. KEY POINTS: Reductions in central nervous system serotonin (CNS5-HT) may contribute to systolic and diastolic dysfunction. This dysfunction may be linked to increases in sympathetic nervous system activity and exacerbated by sex, age and spinal cord injury. Echocardiographic assessment of systolic and diastolic function was completed in young and old male and female intact TPH2+/+ and TPH2-/- mice, as well as in C2 spinal cord hemisected young mice. Lumbar sympathetic nervous system activity was also recorded in elderly male and female intact TPH2+/+ and TPH2-/- mice. Systolic and diastolic dysfunction was evident in young TPH2-/- mice. This dysfunction was exacerbated by age and spinal cord injury. The cardiac dysfunction was accompanied by increases in lumbar sympathetic nervous system activity. Our findings are an initial step toward determining whether reductions in CNS5-HT is a unifying mechanism that links central sleep apnoea, sympathoexcitation and heart failure in intact and spinal cord injured individuals.
Keywords: burst height; burst incidence; echocardiogram; heart failure; lumbar sympathetic nerve activity; mice; serotonin.
© 2025 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Ahmadian, M. , Erskine, E. , Wainman, L. , Wearing, O. H. , Duffy, J. S. , Stewart, L. C. , Hoiland, R. L. , Taki, A. , Perim, R. R. , Mitchell, G. S. , Little, J. P. , Mueller, P. J. , Foster, G. E. , & West, C. R. (2024). Acute intermittent hypoxia elicits sympathetic neuroplasticity independent of peripheral chemoreflex activation and spinal cord tissue hypoxia in a rodent model of high‐thoracic spinal cord injury. Experimental Neurology, 384, 115054. - PubMed
-
- Alenina, N. , Kikic, D. , Todiras, M. , Mosienko, V. , Qadri, F. , Plehm, R. , Boye, P. , Vilianovitch, L. , Sohr, R. , Tenner, K. , Hortnagl, H. , & Bader, M. (2009). Growth retardation and altered autonomic control in mice lacking brain serotonin. Proceedings National Academy of Science USA, 106(25), 10332‐10337. - PMC - PubMed
-
- Barry, W. , St Andre, J. R. , Evans, C. T. , Sabharwal, S. , Miskevics, S. , Weaver, F. M. , & Smith, B. M. (2013). Hypertension and antihypertensive treatment in veterans with spinal cord injury and disorders. Spinal Cord, 51(2), 109–115. - PubMed
-
- Benarroch, E. E. (2014). Medullary serotonergic system: organization, effects, and clinical correlations. Neurology, 83(12), 1104–1111. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
