Insights into the low-temperature adaptation of an enzyme as studied through ancestral sequence reconstruction
- PMID: 39968914
- PMCID: PMC11836894
- DOI: 10.1002/pro.70071
Insights into the low-temperature adaptation of an enzyme as studied through ancestral sequence reconstruction
Abstract
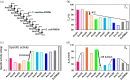
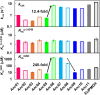
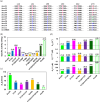
For billions of years, enzymes have evolved in response to the changing environments in which their host organisms lived. Various lines of evidence suggest the earliest primitive organisms inhabited high-temperature environments and possessed enzymes adapted to such conditions. Consequently, extant mesophilic and psychrophilic enzymes are believed to have adapted to lower temperatures during the evolutionary process. Herein, we analyzed this low-temperature adaptation using ancestral sequence reconstruction. Previously, we generated the phylogenetic tree of 3-isopropylmalate dehydrogenases (IPMDHs) and reconstructed the sequence of the last bacterial common ancestor. The corresponding ancestral enzyme displayed high thermostability and catalytic activity at elevated temperatures but moderate activity at low temperatures (Furukawa et al., Sci. Rep., 2020;10:15493). Here, to identify amino acid residues that are responsible for the low-temperature adaptation, we reconstructed and characterized all 11 evolutionary intermediates that sequentially connect the last bacterial common ancestor with extant mesophilic IPMDH from Escherichia coli. A remarkable change in catalytic properties, from those suited for high reaction temperatures to those adapted for low temperatures, occurred between two consecutive evolutionary intermediates. Using a combination of sequence comparisons between ancestral proteins and site-directed mutagenesis analyses, three key amino acid substitutions were identified that enhance low-temperature catalytic activity. Intriguingly, amino acid substitutions that had the most significant impact on activity at low temperatures displayed no discernable effect on thermostability. However, these substitutions markedly reduced the activation energy for catalysis, thereby improving low-temperature activity. The results were further investigated by molecular dynamics simulations of the predicted structures of the ancestral enzymes. Our findings exemplify how ancestral sequence reconstruction can identify residues crucial for adaptation to low temperatures.
Keywords: activation energy; ancestral sequence reconstruction; low‐temperature activity; low‐temperature adaptation; thermostability.
© 2025 The Author(s). Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Bjelic S, Brandsdal BO, Aqvist J. Cold adaptation of enzyme reaction rates. Biochemistry. 2008;47:10049–10057. - PubMed
-
- Busch F, Rajendran C, Heyn K, Schlee S, Merkl R, Sterner R. Ancestral tryptophan synthase reveals functional sophistication of primordial enzyme complexes. Cell Chem Biol. 2016;23:709–715. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

