Intra-class switch among interleukin-17 inhibitors for the treatment of plaque psoriasis: a single-center experience
- PMID: 39969045
- PMCID: PMC12203774
- DOI: 10.4081/dr.2024.10080
Intra-class switch among interleukin-17 inhibitors for the treatment of plaque psoriasis: a single-center experience
Abstract
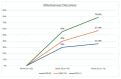
Psoriasis is a chronic immune-mediated disease primarily affecting the skin. The most common subtype is plaque psoriasis, which can affect any body area, with a predilection for the knees, elbows, scalp, lumbosacral region, and genitalia. The European guidelines adopted in Italy recommend systemic therapies for moderate-to-severe psoriasis, defined by a Psoriasis Area and Severity Index (PASI) ≥10, Dermatology Life Quality Index (DLQI) ≥10, and/or Body Surface Area (BSA) ≥10. Over the past two decades, the development of biological agents has revolutionized psoriasis management, targeting specific cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-23, and IL-17. Among these, ixekizumab, secukinumab, brodalumab, and bimekizumab are approved for the treatment of moderate-to-severe plaque psoriasis. However, some patients require switching therapy because of primary/secondary ineffectiveness or side effects. We retrospectively analyzed 20 patients who had switched from one anti-IL-17 drug to another, assessing both safety and effectiveness. At baseline, the median PASI score was 10 (interquartile range [IQR] 4.5). After 16 weeks, it decreased to 2 (IQR 5.5), and after one year, it decreased further to 1 (IQR 2). Eight (40%) and six patients (30%) achieved PASI 90 and PASI 100 at 16 weeks, respectively. After one year, sustained effectiveness was observed, with PASI 90 (57.1%), PASI 100 (35.7%), and PASI≤2 (78.6%). No serious adverse events (AEs) or discontinuations due to AEs were observed during the study period. Our study confirms the safety and effectiveness of intra-class switching among IL-17 antagonists within the same class and highlights that switching between different classes of IL-17 inhibitors can be a valid option when patients fail to respond or lose effectiveness with a particular inhibitor. However, a deeper understanding requires further large-scale and long-term studies.
Conflict of interest statement
LI and LG have been consultants for Almirall; AN has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi-Genzyme, Amgen and Boehringer Ingelheim; AC has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma; MV has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly, Novartis, Janssen, AbbVie and Boehringer Ingelheim, UCB Pharma; GG has no conflict of interet to disclose.
Figures
References
-
- Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis Lancet 2021;397:1301-15. - PubMed
-
- Nast A, Smith C, Spuls PI, et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris - Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol 2020;34:2461-98. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous