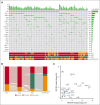
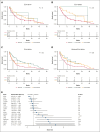
Conflict-of-interest disclosure: A.A.P. received honoraria from AbbVie and Bristol Myers Squibb and institutional research funding from Pfizer and Kronos Bio. R.M.S. received honoraria from Bristol Myers Squibb, Kura Oncology, Gilead Sciences, Rigel, and Servier. E.C.C. received consulting fees from AbbVie and Rigel. S.G.I. received honoraria from Medical Logix (medical education) and reports serving on an advisory board with MorphoSys. R.K.R. received research funding from Incyte, Constellation, Zentalis, Stemline, and Ryyu, as well as consulting fees from Celgene-Bristol Myers Squibb, Kartos, Zentalis, Karyopharm, Dainippon, GlaxoSmithKline-Sierra, Galecto, PharmaEssentia, Incyte, CTI BioPharma, Servier, MorphoSys/Constellation, and Sumitomo. T.B. reports membership on the advisory committee for Novartis, Geron Corporation, and Gilead, as well as participation in the speakers’ bureau for Novartis. Y.A. received research funding from Biomea, Curis, BioSight, ALX Oncology Novartis; and honoraria from Servier, Pfizer, Bristol Myers Squibb, Kite, Astellas, and Rigel. J.S.G. received research funding from AbbVie, Genentech, New Wave, Pfizer, and Prelude; and served on the steering committees and scientific advisory boards of AbbVie, Bristol Myers Squibb, Genentech, and Servier. V.G. received research funding from AbbVie and Novartis; reports consulting fees from Novartis, Bristol Myers Squibb/Celgene, Keros, AbbVie, Constellation Biopharma, Pfizer, GlaxoSmithKline, and CTI BioPharma; reports honoraria from Novartis, Bristol Myers Squibb/Celgene, and AbbVie; and serves on the data safety monitoring or advisory board for Bristol Myers Squibb/Celgene, Roche, AbbVie, Pfizer, GlaxoSmithKline, and CTI BioPharma. K.M.P. reports consulting fees from Protagonist Therapeutics and AbbVie; received research funding from Protagonist Therapeutics, Merck, and AbbVie; and reports speakers bureau membership with Merck. O.O. reports receiving consulting fees from AbbVie, Blueprint Medicines, Bristol Myers Squibb, CTI, Impact Biomedicines, Kymera, Novartis, Servier, Taiho Pharmaceutical, and Treadwell Therapeutics. Additionally, funding for research (to institution) has been received from AbbVie, Agios, Aprea AB, Astex Pharmaceuticals, AstraZeneca, Bristol Myers Squibb, Celgene, CTI BioPharma Corp, Daiichi Sankyo, Incyte, Janssen Oncology, Kartos Therapeutics, Loxo, Novartis, NS Pharma, and OncoTherapy Science. The remaining authors declare no competing financial interests.