LIVE-SMART: A sequential, multiple assignment randomized trial to reduce falls in cirrhosis
- PMID: 39969429
- PMCID: PMC11841856
- DOI: 10.1097/HC9.0000000000000626
LIVE-SMART: A sequential, multiple assignment randomized trial to reduce falls in cirrhosis
Abstract
Introduction: Falls are a major threat to the well-being of patients with cirrhosis. We are performing a clinical trial to determine whether lactulose, TeleTai-Chi, or their combination will reduce falls in HE and improve health-related quality of life (HRQOL) among patients with cirrhosis.
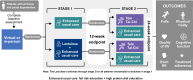
Methods and analysis: Patients with cirrhosis and portal hypertension without HE will be enrolled in 3 US states and followed participants for 24 weeks. In stage 1 (12 wk), participants will be randomized to receive either lactulose therapy or enhanced usual care. In stage 2 (12 wk), participants will be randomized to either TeleTai-Chi or usual care. The primary outcome is a hierarchical composite: Injurious falls, noninjurious falls, incident HE, and death/transplantation. Secondary outcomes include cognitive function, days-alive and out-of-hospital, and HRQOL. After completion of the interventions, participants will be followed for 48 weeks for health and financial outcomes.
Ethics and dissemination: Our study has a central institutional review board with individual site IRB review. Dissemination includes the publication of study findings and patient-focused educational webinars.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Elliot B. Tapper has served as a consultant to Norvartis, Axcella, and Allergan, has served on advisory boards for Mallinckrodt, Bausch Health, Kaleido, Novo Nordisk, and has received unrestricted research grants from Gilead and Valeant. In the last 12 months, Donna Evon has served as a consultant for Hightide Therapeutics, USA. Patricia Bloom has a research grant from Vedanta Biosciences and has served as a consultant for Nexilico. Ethan Weinberg has served as a consultant to Mallinckrodt, Biovie, and PharmaIN. Marina Serper received grants from Grifols and Transplant Genomics. The remaining authors have no conflicts to report.
Figures
References
-
- Mellinger JL, Shedden K, Winder GS, Tapper E, Adams M, Fontana RJ, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology. 2018;68:872–882. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical