Racial, ethnic, and socioeconomic disparities in clinical trial reporting for metastatic spine tumors: An exploration of North American studies
- PMID: 39969615
- PMCID: PMC11839828
- DOI: 10.1007/s10143-025-03343-1
Racial, ethnic, and socioeconomic disparities in clinical trial reporting for metastatic spine tumors: An exploration of North American studies
Abstract
Purpose: The objective of this study was to evaluate the reporting of racial, ethnic, and socioeconomic data in clinical trials exploring the management of metastatic spine disease (MSD).
Methods: We undertook a cross-sectional analysis of North American completed and published clinical trials registered on ClinicalTrials.gov exploring the management of patients with MSD. Data on patient demographics, trial characteristics, reporting of race and ethnicity, distribution of racial and ethnic groups, and reporting of socioeconomic measures was extracted from ClinicalTrials.gov and related publications identified through PubMed and Google Scholar searches. An exploratory data analysis was performed, followed by Pearson's Chi-square and binary logistic regression analyses to explore associations of covariates with racioethnic reporting.
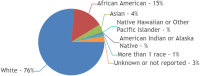
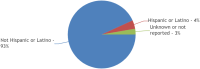
Results: Out of 158 completed trials, only 8% (12 of 158) met inclusion criteria with published results. These 12 trials included a total of 1,568 patients with a mean age of 61 years. Almost half (42%; (5 of 12)) of trials did not report race, while only 17% (2 of 12) of trials reported ethnicity. In trials reporting complete racial data (n = 5), 77% (377 of 493) patients were White, 15% (n = 73) Black or African American, and 4% (n = 19) Asian. American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients were severely underrepresented (0.4% and 0.2%, respectively). Of the two trials reporting ethnicity, 94% (479 of 514) patients were Not Hispanic or Latino. Sponsoring body of the trial, trial phase, intervention type, number of trial patients, or mean age of patients were not significantly associated with racioethnic reporting. Notably, no trial reported any measures of socioeconomic status.
Conclusion: Our review revealed significant gaps in the reporting of racial, ethnic, and socioeconomic data in MSD clinical trials, with substantial underrepresentation of minority groups. This underrepresentation limits the generalizability of trial findings and may perpetuate health disparities. Coordinated efforts from researchers, clinicians, policymakers, and funding bodies are needed to improve diversity in future trials. Strategies such as targeted outreach, community engagement, and more inclusive eligibility criteria should be implemented to ensure that trial populations better reflect the diversity of MSD patients in the general population.
Keywords: Diversity; Employment; Insurance; Metastatic spine disease; Race; Socioeconomic status; Vulnerability.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Being a cross-sectional analysis of published trials, ethics approval was not required to be sought. Competing interests: The authors declare no competing interests. Consent to participate: Being a cross-sectional analysis of published trials, the consent to participate was not required to be sought.
Figures
Similar articles
-
Assessment of the Inclusion of Racial/Ethnic Minority, Female, and Older Individuals in Vaccine Clinical Trials.JAMA Netw Open. 2021 Feb 1;4(2):e2037640. doi: 10.1001/jamanetworkopen.2020.37640. JAMA Netw Open. 2021. PMID: 33606033 Free PMC article. Review.
-
Race and Ethnicity Representation in Phase 2/3 Oncology Clinical Trial Publications: A Systematic Review.JAMA Health Forum. 2024 Jun 7;5(6):e241388. doi: 10.1001/jamahealthforum.2024.1388. JAMA Health Forum. 2024. PMID: 38848090 Free PMC article.
-
Is Our Science Representative? A Systematic Review of Racial and Ethnic Diversity in Orthopaedic Clinical Trials from 2000 to 2020.Clin Orthop Relat Res. 2022 May 1;480(5):848-858. doi: 10.1097/CORR.0000000000002050. Epub 2021 Dec 2. Clin Orthop Relat Res. 2022. PMID: 34855650 Free PMC article.
-
Representation of Race and Ethnicity in Randomized Clinical Trials of Diabetic Macular Edema and Retinal Vein Occlusion Compared to 2010 US Census Data.JAMA Ophthalmol. 2022 Nov 1;140(11):1096-1102. doi: 10.1001/jamaophthalmol.2022.3929. JAMA Ophthalmol. 2022. PMID: 36201192 Free PMC article.
-
Racial and ethnic disparities in clinical trials for pediatric obesity.Obesity (Silver Spring). 2025 Mar;33(3):560-566. doi: 10.1002/oby.24228. Epub 2025 Feb 4. Obesity (Silver Spring). 2025. PMID: 39904717 Free PMC article.
References
-
- Van den Brande R, Cornips EM, Peeters M, Ost P, Billiet C, Van de Kelft E (2022) Epidemiology of spinal metastases, metastatic epidural spinal cord compression and pathologic vertebral compression fractures in patients with solid tumors: a systematic review. J Bone Oncol 35:100446. 10.1016/j.jbo.2022.100446 - PMC - PubMed
-
- Hung B, Pennington Z, Hersh AM et al (2021) Impact of race on nonroutine discharge, length of stay, and postoperative complications after surgery for spinal metastases. J Neurosurg Spine 36(4):678–685. 10.3171/2021.7.SPINE21287 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous