Dysphagia in Parkinson´s disease. A 5-year follow-up study
- PMID: 39969751
- PMCID: PMC12084275
- DOI: 10.1007/s10072-025-08027-8
Dysphagia in Parkinson´s disease. A 5-year follow-up study
Abstract
Background and objective: Dysphagia at time of diagnosis suggests atypical parkinsonism instead Parkinson´s disease (PD). Our aim was to analyze the frequency of dysphagia in patients with early PD comparing with a control group and to identify related factors.
Patients and methods: Patients with early PD (≤ 2 years from symptoms onset) who were recruited from January/2016 to November/2017 (baseline visit; V0) and evaluated annually for 5 years from the Spanish cohort COPPADIS were included in this prospective study. Controls were assessed at baseline and at 2-, 4-, and 5-year follow-up. Dysphagia was defined as a score ≥ 1 in the item 20 of the Non-Motor Symptoms Scale (NMSS).
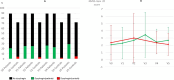
Results: Dysphagia was more frequent at baseline in PD patients (19.6% [36/184]; 62.3 ± 8.3 years old; 56.8% males) than in controls (5.3% [11/206]; 60.9 ± 8.3 years old; 50% males) (p < 0.0001) and in all visits as well (p < 0.0001). A worse quality of sleep (Parkinson´s Disease Sleep Scale; OR = 0.974; p = 0.005), a greater impulse-control behavior (ICB) (Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease-Rating Scale; OR = 1.066; p = 0.014), and non-motor symptoms burden (Non-Motor Symptoms Scale; OR = 1.016; p = 0.021) were independent factors associated with dysphagia at baseline. In those subjects with dysphagia, no differences were observed between patients and controls in the mean NMSS-item 20 overtime, and it didn´t change throughout the follow-up.
Conclusion: Dysphagia was frequent in early PD patients compared to controls. However, it was minor and did not progress over time. Sleep, ICB, and non-motor symptoms burden were related to dysphagia.
Keywords: Cohort; Dysphagia; Early; Impulse control disorder; Non-motor symptoms; Parkinson's disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Financial disclosures for the previous 12 months: Diego Santos-García has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, Italfarmaco, Teva, Archímedes, Esteve, Stada, Merz, and grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Concesión de subvenciones de Proyectos de Investigación en Salud de la convocatoria 2020 de la Acción Estratégica en Salud 2017–2020 por el proyecto “PROGRESIÓN NO MOTORA E IMPACTO EN LA CALIDAD DE VIDA EN LA ENFERMEDAD DE PARKINSON”). Teresa de Deus: None. Silvia Jesús has received honoraria from AbbVie, Bial, Merz, UCB, and Zambon and holds the competitive contract "Juan Rodés" supported by the Instituto de Salud Carlos III. She has received grants from the Spanish Ministry of Economy and Competitiveness (PI18/01898) and the Consejería de Salud de la Junta de Andalucía (PI-0459–2018). Marina Cosgaya: None. Juan García Caldentey has received honoraria for educational presentations and advice service by Qualigen, Nutricia, Abbvie, Italfarmaco, UCB Pharma, Lundbeck, Zambon, Bial, and Teva. Nuria Caballol has received honoraria from Bial, Italfármaco, Qualigen, Zambon, UCB, Teva and KRKA and sponsorship from Zambon, TEVA and Abbvie for attending medical conferences. Ines Legarda has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva. Jorge Hernández Vara has received travel bursaries and educational grants from Abbvie and has received honoraria for educational presentations from Abbvie, Teva, Bial, Zambon, Italfarmaco, and Sanofi-Genzyme. Iria Cabo López: has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial. Lydia López Manzanares compensated advisory services, consulting, research grant support, or speaker honoraria: AbbVie, Acorda, Bial, Intec Pharma, Italfarmaco, Pfizer, Roche, Teva, UCB, and Zambon. Isabel González Aramburu: None. Asunción Ávila Rivera has received honoraria from Zambon, UCB Pharma, Qualigen, Bial, and Teva, and sponsorship from Zambon and Teva for attending conferences. Víctor Gómez Mayordomo has received honoraria from Bial, Merz and Zambon for educational lectures. Víctor Nogueira: None. Julio Dotor García-Soto compensated advisory services, consulting, research grant support, or speaker honoraria: Merck, Sanofi-Genzyme, Allergan, Biogen, Roche, UCB and Novartis. Carmen Borrué-Fernández: None. Berta Solano has received honoraria for educational presentations and advice service by UCB, Zambon, Teva, Abbvie, Bial. María Álvarez Sauco has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva. Lydia Vela has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva. Sonia Escalante has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial. Esther Cubo: Travel grants: Abbvie, Allergan, Boston; Lecturing honoraria: Abbvie, International Parkinson´s disease Movement Disorder Society. Zebenzui Mendoza: None. Isabel Pareés has received honoraria as speaker in scientific meetings from Zambon, Abbvie, Bial, Exeltis Healthcare SL, Sociedad Extremeña de Neurología, Sociedad Española de Neurología and travel support for scientific meetings from Esteve. Pilar Sánchez Alonso has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva. Maria G. Alonso Losada has received honoraria for educational presentations and advice service by Zambon and Bial. Nuria López Ariztegui has received honoraria for educational presentations and advice service or travel grants by Abbvie, Italfarmaco, Stada, Lundbeck, UCB, Esteve, Abbott, Zambon, and Bial. Itziar Gastón has received research support from Abbvie and Zambon and has served as a consultant for Abbvie, Exelts, and Zambon. Jaime Kulisevsky: (1) Consulting fees: Roche, Zambon; (2) Stock / allotment: No; (3) Patent royalties / licensing fees: No; (4) Honoraria (e.g. lecture fees): Zambon, Teva, Bial, UCB; (5) Fees for promotional materials: No; (6) Research funding: Roche, Zambon, Ciberned; Instituto de SaludCarlos III; FundacióLa Maratóde TV3; (7) Scholarship from corporation: No; (8) Corporate laboratory funding: No; (9) Others (e.g. trips, travel, or gifts): No. Manuel Seijo has received honoraria for educational services from KRKA, UCB, Zambon, Bial; travel grants from Daiichi and Roche. Caridad Valero has received honoraria for educational services from Zambon, Abbvie and UCB. Ruben Alonso Redondo: None. Maria Teresa Buongiorno: None. Carlos Ordás: None. Manuel Menéndez-González has received consulting honoraria from Eisai Spain and research grants from by Instituto de Salud Carlos III (ISCIII), Fondo Europeo de Desarrollo Regional (FEDER), and Fundación Alimerka. Darrian McAfee: None. Pablo Martinez-Martin has received honoraria from the Parkinson and Movement Disorder Society (MDS) for management of the COA International Program of the Society. Pablo Mir has received honoraria from AbbVie, Abbott, Allergan, Bial, Merz, UCB, and Zambon and have received grants from the Spanish Ministry of Economy and Competitiveness [ PI16/01575] co-founded by ISCIII (Subdirección General de Evaluación y Fomento de la Investigación) and by Fondo Europeo de Desarrollo Regional (FEDER), the Consejería de Economía, Innovación, Ciencia y Empleo de la Junta de Andalucía [CVI-02526, CTS-7685], the Consejería de Salud y Bienestar Social de la Junta de Andalucía [ PI-0437–2012, PI-0471–2013], the Sociedad Andaluza de Neurología, the Jacques and Gloria Gossweiler Foundation, the Fundación Alicia Koplowitz, the Fundación Mutua Madrileña. Ethical aspects: For this study, we received approval from the Comité de Ética de la Investigación Clínica de Galicia from Spain (2014/534; 02/DEC/2014). Written informed consents from all participants in this study were obtained. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. The protocol and the statistical analysis plan are available on request. Deidentified participant data are not available for legal and ethical reasons. Ethical approval and informed consent: For this study, the consent form and the approval can be found at https://doi.org/10.1007/s10072-025-08027-8 . Conflict of interest: None.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

