Mussel-inspired cross-linking mechanisms enhance gelation and adhesion of multifunctional mucin-derived hydrogels
- PMID: 39969995
- PMCID: PMC11874598
- DOI: 10.1073/pnas.2415927122
Mussel-inspired cross-linking mechanisms enhance gelation and adhesion of multifunctional mucin-derived hydrogels
Abstract
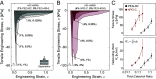
Mucus supports human health by hydrating, lubricating, and preventing infection of wet epithelial surfaces. The beneficial material properties and bioactivity of mucus stem from glycoproteins called mucins, motivating the development of mucin-derived hydrogels for wound dressings and antifouling coatings. However, these applications require robust gelation and adhesion to a wide range of substrates. Inspired by the chemical cross-linking and water-tolerant adhesion of marine mussel adhesive structures, we use catechol-thiol bonding to drive gelation of native mucin proteins and synthetic mucin-inspired polymers, forming soft, adhesive hydrogels that can be coated onto diverse surfaces. The gelation dynamics and adhesive properties can be systematically tuned by varying the hydrogel composition, polymer architecture, and thiol availability, with gelation timescales adjustable from seconds to hours, and values of elastic modulus, failure stress, and debonding work spanning orders of magnitude. We demonstrate the functionality of these gels in two applications: as tissue adhesives, using porcine skin as a proxy for human skin, and as bioactive surface coatings to prevent bacterial colonization. The results highlight the potential of catechol-thiol cross-linking as a versatile platform for engineering multifunctional glycoprotein hydrogels with applications in wound repair and antimicrobial surface engineering.
Keywords: antifouling; biomaterials; catechol-thiol bond; mucus; tissue adhesive.
Conflict of interest statement
Competing interests statement:G.D.D., C.A.S., K.R., and G.H.M. are inventors on a provisional US patent application (63/639,581) covering a portion of this work.
Figures




References
-
- Petrou G., Crouzier T., Mucins as multifunctional building blocks of biomaterials. Biomater. Sci. 6, 2282–2297 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

