First-line anti-TNF agents, ustekinumab and vedolizumab perform similarly in Crohn' disease, but not in ulcerative colitis
- PMID: 39970039
- PMCID: PMC11949238
- DOI: 10.1097/MEG.0000000000002940
First-line anti-TNF agents, ustekinumab and vedolizumab perform similarly in Crohn' disease, but not in ulcerative colitis
Abstract
Background: Real-word comparisons between first-line biologicals in inflammatory bowel disease (IBD) are scarce.
Aims: The aim of this study is to compare drug persistence and patient reported outcome-2 (PRO-2) remission rates of first-line biological classes [anti-tumor necrosis factor (TNF) agents vs anti-integrin vedolizumab vs IL-12/23 inhibitor ustekinumab] in real life cohort.
Methods: Individual level data of 946 adults (588 Crohn's disease and 358 ulcerative colitis) were retrieved from UR-CARE IBD platform. Adjusted drug survival curves using a pooled logistic model and PRO-2 remission rates for each class of biologicals were calculated and compared.
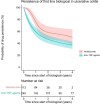
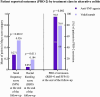
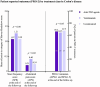
Results: In Crohn's disease, no differences in drug survival were observed for anti-TNF agents vs vedolizumab vs ustekinumab as estimated survival with 95% confidence intervals were 0.81 (0.77-0.84) vs 0.89 (0.82-0.96) vs 0.88 (0.79-0.97) at year 1 and 0.52 (0.46-0.58) vs 0.58 (0.37-0.78) vs 0.58 (0.39-0.77) at year 4. In ulcerative colitis, however, anti-TNF agents had shorter drug survival than vedolizumab with estimated drug survival with 95% confidence intervals 0.60 (0.52-0.67) vs 0.76 (0.67-0.84) at year 1 and 0.37 (0.30-0.44) vs 0.50 (0.36-0.64) at year 4. No differences in PRO-2 remission rates were observed between drug classes in Crohn's disease ( P = 0.95), but more patients enjoyed PRO-2 remission in ulcerative colitis treated with anti-TNF agents compared to vedolizumab (94.8 vs 78.9%, P = 0.002).
Conclusion: Our real-world data suggest similar drug persistence and efficacy of first-line treatments with anti-TNF agents, vedolizumab and ustekinumab in Crohn's disease. In ulcerative colitis, however, drug persistence was higher for vedolizumab compared to anti-TNF agents, but on the cost of lower PRO-2 remission rates.
Keywords: biological drugs; clinical remission; drug persistence; inflammatory bowel disease; patient reported outcome.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
J.H. has served as a consultant for Abbvie, Alimentiv Inc., Janssen, and Takeda. G.N. has served as a speaker, consultant, and advisory board member for Abbvie, Takeda, Pfizer, Janssen, Oktal Pharma, Sobi, Krka, Sandoz, and Biogen. B.Š. served as a speaker, a consultant, and/or an advisory board member for MSD, Abbvie, Takeda, Pfizer, and Janssen. D.D. has served as a speaker, consultant, and/or advisory board member for MSD, Abbvie, Takeda, Pfizer, Janssen, Krka, Eli Lilly, Oktal Pharma, Roche, Novartis, Amgen, and Lek. For the remaining authors, there are no conflicts of interest.
Figures


References
-
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. . Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017; 390:2769–2778. - PubMed
-
- Turner D, Ricciuto A, Lewis A, D’Amico F, Dhaliwal J, Griffiths AM, et al. . STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160:1570–1583. - PubMed
-
- Baumgart DC, Le Berre C. Newer biologic and small-molecule therapies for inflammatory bowel disease. N Engl J Med. 2021; 385:1302–1315. - PubMed
-
- Sands BE, Peyrin-Biroulet L, Loftus EV, Danese S, Colombel JF, Törüner M, et al. . Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019; 381:1215–1226. - PubMed
-
- Sands BE, Irving PM, Hoops T, Izanec JL, Gao LL, Gasink C, et al. . Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn’s disease: a multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet. 2022; 399:2200–2211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

