The mutational landscape and functional effects of noncoding ultraconserved elements in human cancers
- PMID: 39970212
- PMCID: PMC11837999
- DOI: 10.1126/sciadv.ado2830
The mutational landscape and functional effects of noncoding ultraconserved elements in human cancers
Abstract
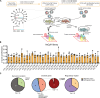
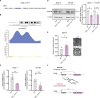
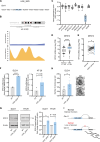
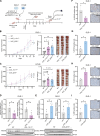
The mutational landscape of phylogenetically ultraconserved elements (UCEs), especially those in noncoding DNAs (ncUCEs), and their functional relevance in cancers remain poorly characterized. Here, we perform a systematic analysis of whole-genome and in-house targeted UCE sequencing datasets from more than 3000 patients with cancer of 13,736 UCEs and demonstrate that ncUCE somatic alterations are common. Using a multiplexed CRISPR knockout screen in colorectal cancer cells, we show that the loss of several altered ncUCEs significantly affects cell proliferation. In-depth functional studies in vitro and in vivo further reveal that specific ncUCEs can be enhancers of tumor suppressors (such as ARID1B) and silencers of oncogenic proteins (such as RPS13). Moreover, several miRNAs located in ncUCEs are recurrently mutated. Mutations in miR-142 locus can affect the Drosha-mediated processing of precursor miRNAs, resulting in the down-regulation of the mature transcript. These results provide systematic evidence that specific ncUCEs play diverse regulatory roles in cancer.
Figures


References
-
- Calin G. A., Trapasso F., Shimizu M., Dumitru C. D., Yendamuri S., Godwin A. K., Ferracin M., Bernardi G., Chatterjee D., Baldassarre G., Rattan S., Alder H., Mabuchi H., Shiraishi T., Hansen L. L., Overgaard J., Herlea V., Mauro F. R., Dighiero G., Movsas B., Rassenti L., Kipps T., Baffa R., Fusco A., Mori M., Russo G., Liu C.-G., Neuberg D., Bullrich F., Negrini M., Croce C. M., Familial cancer associated with a polymorphism in ARLTS1. N. Engl. J. Med. 352, 1667–1676 (2005). - PubMed
-
- Stark R., Grzelak M., Hadfield J., RNA sequencing: The teenage years. Nat. Rev. Genet. 20, 631–656 (2019). - PubMed
-
- Zehir A., Benayed R., Shah R. H., Syed A., Middha S., Kim H. R., Srinivasan P., Gao J., Chakravarty D., Devlin S. M., Hellmann M. D., Barron D. A., Schram A. M., Hameed M., Dogan S., Ross D. S., Hechtman J. F., DeLair D. F., Yao J., Mandelker D. L., Cheng D. T., Chandramohan R., Mohanty A. S., Ptashkin R. N., Jayakumaran G., Prasad M., Syed M. H., Rema A. B., Liu Z. Y., Nafa K., Borsu L., Sadowska J., Casanova J., Bacares R., Kiecka I. J., Razumova A., Son J. B., Stewart L., Baldi T., Mullaney K. A., Al-Ahmadie H., Vakiani E., Abeshouse A. A., Penson A. V., Jonsson P., Camacho N., Chang M. T., Won H. H., Gross B. E., Kundra R., Heins Z. J., Chen H. W., Phillips S., Zhang H., Wang J., Ochoa A., Wills J., Eubank M., Thomas S. B., Gardos S. M., Reales D. N., Galle J., Durany R., Cambria R., Abida W., Cercek A., Feldman D. R., Gounder M. M., Hakimi A. A., Harding J. J., Iyer G., Janjigian Y. Y., Jordan E. J., Kelly C. M., Lowery M. A., Morris L. G. T., Omuro A. M., Raj N., Razavi P., Shoushtari A. N., Shukla N., Soumerai T. E., Varghese A. M., Yaeger R., Coleman J., Bochner B., Riely G. J., Saltz L. B., Scher H. I., Sabbatini P. J., Robson M. E., Klimstra D. S., Taylor B. S., Baselga J., Schultz N., Hyman D. M., Arcila M. E., Solit D. B., Ladanyi M., Berger M. F., Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 23, 703–713 (2017). - PMC - PubMed
MeSH terms
Substances
Grants and funding
- U24 CA264128/CA/NCI NIH HHS/United States
- U01 CA253472/CA/NCI NIH HHS/United States
- R01 DE032018/DE/NIDCR NIH HHS/United States
- R01 CA251150/CA/NCI NIH HHS/United States
- R01 GM122775/GM/NIGMS NIH HHS/United States
- U54 CA096300/CA/NCI NIH HHS/United States
- P50 CA221703/CA/NCI NIH HHS/United States
- R01 CA182905/CA/NCI NIH HHS/United States
- P50 CA127001/CA/NCI NIH HHS/United States
- U01 CA281902/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- R01 CA222007/CA/NCI NIH HHS/United States
- U54 CA096297/CA/NCI NIH HHS/United States
- P50 CA281701/CA/NCI NIH HHS/United States

