Low-Dose Yellow Fever Vaccine in Adults in Africa
- PMID: 39970397
- PMCID: PMC7617464
- DOI: 10.1056/NEJMoa2407293
Low-Dose Yellow Fever Vaccine in Adults in Africa
Abstract
Background: Yellow fever vaccine is highly effective with a single dose, but vaccine supply is limited. The minimum dose requirements for seroconversion remain unknown.
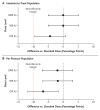
Methods: In this double-blind, randomized, noninferiority trial in Uganda and Kenya, we assigned adults with no history of yellow fever vaccination or infection to receive vaccination with the Institut Pasteur de Dakar 17D-204 yellow fever vaccine at a standard dose (13,803 IU) or at a fractional dose of 1000 IU, 500 IU, or 250 IU. The primary outcome was seroconversion at 28 days after vaccination with each fractional dose as compared with the standard dose, evaluated in a noninferiority analysis. Seroconversion was defined as an antibody titer at day 28 that was at least four times as high as the antibody titer before vaccination, as measured by a plaque reduction neutralization test. We conducted noninferiority analyses in the per-protocol and intention-to-treat populations. Noninferiority was shown if the lower boundary of the 95% confidence interval for the difference in the incidence of seroconversion between the fractional dose and the standard dose was higher than -10 percentage points.
Results: A total of 480 participants underwent randomization (120 participants in each group). The incidence of seroconversion was 98% (95% confidence interval [CI], 94 to 100) with the standard dose. The difference in the incidence of seroconversion between the 1000-IU dose and the standard dose was 0.01 percentage points (95% CI, -5.0 to 5.1) in the intention-to-treat population and -1.9 percentage points (95% CI, -7.0 to 3.2) in the per-protocol population; the corresponding differences between the 500-IU dose and the standard dose were 0.01 percentage points (95% CI, -5.0 to 5.1) and -1.8 percentage points (95% CI, -6.7 to 3.2), and those between the 250-IU dose and the standard dose were -4.4 percentage points (95% CI, -9.4 to 0.7) and -6.7 percentage points (95% CI, -11.7 to 1.6). A total of 111 vaccine-related adverse events were reported: 103 were mild in severity, 7 were moderate, and 1 was severe. The incidence of adverse events was similar in the four groups.
Conclusions: A yellow fever vaccination dose as low as 500 IU was noninferior to the standard dose of 13,803 IU for producing seroconversion within 28 days. (Funded by the European and Developing Countries Clinical Trials Partnership and the Wellcome Trust; NIFTY ClinicalTrials.gov number, NCT04059471.).
Copyright © 2025 Massachusetts Medical Society.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
