Transformative approaches for effective clinical trials to reduce the disease burden of osteoarthritis
- PMID: 39970622
- PMCID: PMC12433614
- DOI: 10.1016/j.semarthrit.2025.152652
Transformative approaches for effective clinical trials to reduce the disease burden of osteoarthritis
Abstract
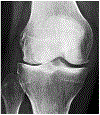
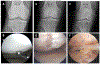
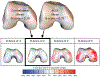
Osteoarthritis (OA) is a leading cause of disability and morbidity that has eluded development of effective disease modifying drugs and therapies. While established OA in the form of symptomatic radiographic disease is a recognizable final common pathway, OA development encompasses a broad spectrum of pathological changes, susceptibilities, and etiological pathways that cannot be considered a single disease process. Beginning with preclinical disease where radiographs are normal, the concept of pre-osteoarthritis (pre-OA) offers a systems-based approach to OA prevention by targeting reduction of OA risk prior to the onset of definable OA. Early OA ensues when cellular, molecular, and joint tissue changes begin to overlap that of OA, a process that can begin before the onset of definitive symptoms or radiographic changes. A myriad of pathways and crossroads of pre-OA and early OA eventually leads to poorly irreversible symptomatic radiographic OA. With increasing recognition of pre-OA and early OA markers, pathways and subtypes, opportunities arise to address these new therapeutic targets. The current status of clinical trials in OA was identified as a critical barrier to progress by the 2022 National Institute of Arthritis, Musculoskeletal, and Skin Diseases (NIAMS) Roundtable on "Cartilage Preservation and Restoration in Knee Osteoarthritis: Challenges, Gaps, and Opportunities". This manuscript summarizes the recommendations of the work group established from the Roundtable to address this issue. The work group recommends that clinical trial design and endpoints evolve to effectively evaluate new treatment approaches suitable for pre-osteoarthritis and early OA by different criteria than what has been set for symptomatic radiographic OA. While symptomatic improvement is the primary goal for palliation of irreversible established OA, important goals for treating earlier disease states include disease modification and prevention, with the potential to alter the natural history of progressive OA. Because symptoms may not correlate with structural changes in pre-OA and early OA, the primary outcomes in these trials need to match the intended mechanistic target and the therapeutic goal for the disease state being treated. The purpose of this manuscript is to transform the approach to clinical trials in OA by establishing a new benchmark of identifying critical outcomes that are appropriate for the joint disease states and subtypes of the target patient population, and the therapeutic or mechanistic target of the intervention being tested. By shifting the approach from using standardized outcomes based on established OA towards customizing clinical trials according to these principles, new precision medicine strategies to address the full spectrum of disease from pre-OA to OA can be more readily advanced into clinical practice.
Keywords: Biologics; Cartilage; Clinical trials; Early OA; MRI; Precision medicine; Preosteoarthritis.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Constance Chu reports research MRI sequence support from GE Healthcare. Farshid Guilak reports a relationship with Cytex Therapeutics that includes: board membership and employment. Christian Lattermann reports a relationship with Vericel Corporation that includes: consulting or advisory. Christian Lattermann reports a relationship with Organogenesis Inc that includes: consulting or advisory. Scott Rodeo reports consulting relationships with Novartis and Advance Medical, stock options with Jannu Therapeutics, Inc and Overture Medical, and research support from Angiocrine Biosciences, Inc. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Yang G, Wang J, Liu Y. Burden of Knee Osteoarthritis in 204 countries and territories, 1990-2019: results from the global burden of disease study 2019. Arthritis Care Res 2023;75(12):2489–500 (Hoboken). - PubMed
-
- Altman R, Alarcon G, Appelrouth D. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum 1991;34(5):505–14. - PubMed
-
- Altman R, Asch E, Bloch D. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the American rheumatism association. Arthritis Rheum 1986;29(8):1039–49. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

