Vitamin E (300 mg) in the treatment of MASH: A multi-center, randomized, double-blind, placebo-controlled study
- PMID: 39970876
- PMCID: PMC11866479
- DOI: 10.1016/j.xcrm.2025.101939
Vitamin E (300 mg) in the treatment of MASH: A multi-center, randomized, double-blind, placebo-controlled study
Abstract
The efficacy and safety of a lower dose of vitamin E for metabolic dysfunction-associated steatohepatitis (MASH) treatment are unclear. This multi-center, randomized, double-blind, placebo-controlled study includes 124 non-diabetic participants with biopsy-proven MASH. Participants are randomly assigned to receive oral vitamin E 300 mg or the placebo in a 1:1 ratio. The primary outcome is improvement in hepatic histology. In the modified intention-to-treat population, 29.3% of participants in the vitamin E group achieve the primary outcome compared with 14.1% in the placebo group. Significant improvement in steatosis, lobular inflammation, and fibrosis stages is observed in the vitamin E group. 12 serious adverse events are reported in this trial but are not considered to be related to the treatment. Vitamin E 300 mg daily achieves sound improvements in liver histology in the Chinese population with MASH. This study is registered at ClinicalTrials.gov (NCT02962297).
Keywords: clinical trial; liver fibrosis; metabolic dysfunction-associated steatohepatitis; metabolic dysfunction-associated steatotic liver disease; non-alcoholic fatty liver disease; vitamin E.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

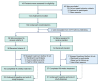
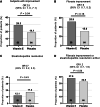

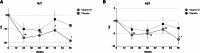
References
-
- Li J., Zou B., Yeo Y.H., Feng Y., Xie X., Lee D.H., Fujii H., Wu Y., Kam L.Y., Ji F., et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet. Gastroenterol. Hepatol. 2019;4:389–398. doi: 10.1016/S2468-1253(19)30039-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

