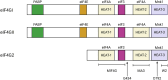
eIF3d and eIF4G2 mediate an alternative mechanism of cap-dependent but eIF4E-independent translation initiation
- PMID: 39971159
- PMCID: PMC11968281
- DOI: 10.1016/j.jbc.2025.108317
eIF3d and eIF4G2 mediate an alternative mechanism of cap-dependent but eIF4E-independent translation initiation
Abstract
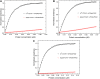
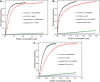
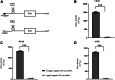
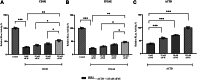
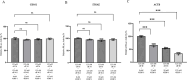
Initiation of translation for the majority of eukaryotic mRNAs is mediated by a 5' cap structure to which the eukaryotic initiation factor 4E (eIF4E) binds. Inhibition of the activity of eIF4E by 4EBP-1 does not prevent the translation of a number of cellular capped mRNAs, indicative of the existence of previously unexplored mechanisms for the translation of these capped mRNAs without the requirement of eIF4E. eIF4G2, also known as death-associated protein 5 (DAP5), a homolog of eIFGI that lacks the eIF4E binding domain, utilizes eIF3d (a subunit of eIF3) to promote the translation of a subset of these mRNAs. Using fluorescence anisotropy-based equilibrium binding studies, we provide the first quantitative evidence of the recruitment of eIF3d as well as eIF3d and eIFG2 complexes to a subset of human mRNAs. Our quantitative studies demonstrate the critical role a fully methylated 5' mRNA cap structure plays in the recognition and recruitment of eIF3d, as well as the eIF3d and eIFG2 complex. By using luciferase reporter-based in vitro translation assays, we further show that cap-recognition ability correlates with the efficiency of translation of these mRNAs. Essentially, by preferably utilizing eIF3d and eIFG2, specific mRNA subsets are still able to translate in a cap-dependent manner even when eIF4E is sequestered. Our findings offer new insight into the use of eIF3d and eIF4G2 as an alternative for growth and survival under conditions of cellular stress. This novel mechanism of translation may offer new targets for therapeutic regulation of mRNA translation.
Keywords: 7-methylguanosine cap (m(7)G) cap; eIF3d; eIF4G2; fluorescence anisotropy; hypoxia.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



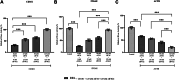


References
-
- Brito Querido J., Díaz-López I., Ramakrishnan V. The molecular basis of translation initiation and its regulation in eukaryotes. Nat. Rev. Mol. Cell Biol. 2024;25:168–186. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

