Micro-CT analysis reveals porosity driven growth banding in Caribbean coral Siderastrea siderea
- PMID: 39971762
- PMCID: PMC11840155
- DOI: 10.1038/s41598-025-90125-w
Micro-CT analysis reveals porosity driven growth banding in Caribbean coral Siderastrea siderea
Abstract
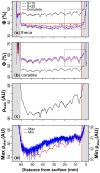
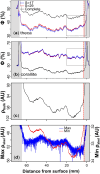
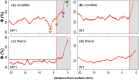
X-radiography of massive scleractinian coral skeletons reveal light and dark couplets termed "growth bands", which are commonly related to seasonal fluctuations in environmental parameters including insolation and sea surface temperature (SST). Massive corals grow by extension of skeletal structures followed by thickening within the surface tissue layer. Therefore, an understanding of the depth in which skeletal thickening occurs is important to aid the interpretation of seasonal banding patterns. In this study, two colonies of Caribbean coral Siderastrea siderea were sampled from the north-west coast of Barbados at water depths of 5 and 15 m. The three-dimensional skeletal structure of each sample was reconstructed at high spatial resolutions using micro-computed tomography (µCT) scanning. A pixel segmentation algorithm was developed to classify different microstructures within the skeleton and to quantify spatial variations in corallite and theca porosity at the micrometer scale. The porosity reconstructions of the deeper sample reveal clearer growth banding, with a more dominant signal originating from within the corallite. Skeletal thickening occurs within the top two-thirds of the total depth of soft tissues and the rate of thickening varies between microstructures. Seasonality in the shallower sample is less clear, although porosity variability with depth is more similar across microstructures. The difference in signal origin and clarity between the two samples is attributed to the varying stability of water depth-dependent variables (i.e., insolation and wave energy). This study provides a new, powerful method of reconstructing and understanding growth strategies in massive scleractinian corals.
Keywords: Caribbean; Density banding; Scleractinian; Seasonality; Segmentation; Tomography.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Consent for publication: All authors have read and approved the final version of the manuscript and consent to its submission for publication. Samples were imported with CITES permit number 24EB000707-AS.
Figures




References
-
- Barnes, D. J. & Lough, J. M. Systematic variations in the depth of skeleton occupied by coral tissue in massive colonies of Porites from the Great barrier reef. J. Exp. Mar. Biol. Ecol.159(1), 113–128. 10.1016/0022-0981(92)90261-8 (1992).
-
- Mollica, N. R. et al. Skeletal records of bleaching reveal different thermal thresholds of Pacific coral reef assemblages. Coral. Reefs38(4), 743–757. 10.1007/s00338-019-01803-x (2019).
-
- DeCarlo, T. M. & Cohen, A. L. Dissepiments, density bands and signatures of thermal stress in Porites skeletons. Springer10.1007/s00338-017-1566-9 (2017).
-
- Tierney, J. E. et al. Tropical sea surface temperatures for the past four centuries reconstructed from coral archives. Paleoceanography30(3), 226–252. 10.1002/2014PA002717 (2015).
-
- Tanzil, J. T. I. et al. Luminescence and density banding patterns in massive Porites corals around the Thai-Malay Peninsula, Southeast Asia. Limnol Oceanogr61(6), 2003–2026. 10.1002/lno.10350 (2016).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

