Sephin1 suppresses ER stress-induced cell death by inhibiting the formation of PP2A holoenzyme
- PMID: 39971896
- PMCID: PMC11840111
- DOI: 10.1038/s41419-025-07450-1
Sephin1 suppresses ER stress-induced cell death by inhibiting the formation of PP2A holoenzyme
Abstract
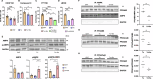
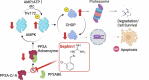
Sephin1 was discovered as a protein phosphatase inhibitor, and its efficacy against neurodegenerative diseases has been confirmed. There are conflicting reports on whether inhibition of eIF2α dephosphorylation by PP1 holoenzyme with the protein phosphatase 1 regulatory subunit 15 A is the mechanism of action of Sephin1. In the present study, we found that Sephin1 significantly suppressed renal tubular cell death in an animal model of ER stress administered with tunicamycin. CHOP, which plays a central role in the ER stress-induced cell death pathway, requires nuclear translocation to act as a transcription factor to increase the expression of cell death-related genes. Sephin1 markedly suppressed this nuclear translocation of CHOP. To elucidate the molecular mechanism underlying the cell death suppressive effect of Sephin1, we used human renal tubular epithelial cells under ER stress with tunicamycin. Sephin1 reduced intracellular CHOP levels by promoting CHOP phosphorylation at Ser30, which led to protein degradation in UPS. Phosphorylated CHOP is generated by Thr172-phosphorylated activated AMPK, and Sephin1 increased phosphorylated AMPK. Phosphorylated AMPK is inactivated by PP2A through dephosphorylation of its Thr172, and Sephin1 inhibits the formation of the PP2A holoenzyme with the PP2A subunit B isoform delta. These results indicate that inhibition of PP2A holoenzyme formation is the molecular target of Sephin1 in this experimental system.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: SG is a Scientific advisor to Remiges Ventures, Inc., and he has a patent related to this work. Ethics: The animal study was reviewed and approved by the Animal Experiment Ethics Committee of Kyoto Prefectural University of Medicine (Approval number, M2022-530).
Figures




References
-
- Tsaytler P, Bertolotti A. Exploiting the selectivity of protein phosphatase 1 for pharmacological intervention. FEBS J. 2013;280:766–70. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

