Ball-and-chain inactivation of a human large conductance calcium-activated potassium channel
- PMID: 39971906
- PMCID: PMC11840039
- DOI: 10.1038/s41467-025-56844-4
Ball-and-chain inactivation of a human large conductance calcium-activated potassium channel
Abstract
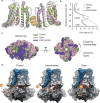
BK channels are large-conductance calcium (Ca2+)-activated potassium channels crucial for neuronal excitability, muscle contraction, and neurotransmitter release. The pore-forming (α) subunits co-assemble with auxiliary (β and γ) subunits that modulate their function. Previous studies demonstrated that the N-termini of β2-subunits can inactivate BK channels, but with no structural correlate. Here, we investigate BK β2-subunit inactivation using cryo-electron microscopy, electrophysiology and molecular dynamics simulations. We find that the β2 N-terminus occludes the pore only in the Ca2+-bound open state, via a ball-and-chain mechanism. The first three hydrophobic residues of β2 are crucial for occlusion, while the remainder of the N-terminus remains flexible. Neither the closed channel conformation obtained in the absence of Ca2+ nor an intermediate conformation found in the presence of Ca2+ show density for the N-terminus of the β2 subunit in their pore, likely due to narrower side access portals preventing their entry into the channel pore.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



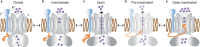
References
-
- Pallotta, B. S., Magleby, K. L. & Barrett, J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature293, 471–474 (1981). - PubMed
-
- Latorre, R., Oberhauser, A., Labarca, P. & Alvarez, O. Varieties of calcium-activated potassium channels. Annu. Rev. Physiol.51, 385–399 (1989). - PubMed
-
- Calderone, V. Large-conductance, ca(2+)-activated k(+) channels: function, pharmacology and drugs. Curr. Med. Chem.9, 1385–1395 (2002). - PubMed
-
- Kaczorowski, G. J., Knaus, H. G., Leonard, R. J., McManus, O. B. & Garcia, M. L. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J. Bioenerg. Biomembr.28, 255–267 (1996). - PubMed
MeSH terms
Substances
Grants and funding
- P30 CA016087/CA/NCI NIH HHS/United States
- F32 GM145091/GM/NIGMS NIH HHS/United States
- R01 GM088352/GM/NIGMS NIH HHS/United States
- R01 GM128420/GM/NIGMS NIH HHS/United States
- GM128420/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- F32GM145091/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- GM088352/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- Postdoctoral fellowship/Hartwell Foundation (The Hartwell Foundation)
LinkOut - more resources
Full Text Sources
Miscellaneous

