Metformin inhibits the growth of SCLC cells by inducing autophagy and apoptosis via the suppression of EGFR and AKT signalling
- PMID: 39971923
- PMCID: PMC11839993
- DOI: 10.1038/s41598-025-87537-z
Metformin inhibits the growth of SCLC cells by inducing autophagy and apoptosis via the suppression of EGFR and AKT signalling
Abstract
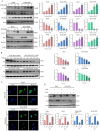
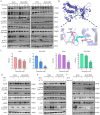
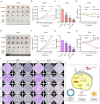
Small cell lung cancer (SCLC) is a therapeutically challenging disease. Metformin, an effective agent for the treatment of type 2 diabetes, has been shown to have antitumour effects on many cancers, including non-small cell lung cancer (NSCLC) and breast cancer. Currently, the antitumour effects of metformin on SCLC and the underlying molecular mechanisms remain unclear. CCK-8, EdU, colony formation, flow cytometry, immunofluorescence, molecular docking, western blotting, nude mouse transplanted tumour model, and immunohistochemistry experiments were conducted to analyse gene functions and the underlying mechanism involved. In vitro experiments demonstrated that metformin inhibited the growth of SCLC cells (H446, H526, H446/DDP and H526/DDP), which was confirmed in xenograft mouse models in vivo. Additionally, metformin induced cell cycle arrest, apoptosis, and autophagy in these SCLC cells. The molecular docking results indicated that metformin has a certain binding affinity for EGFR. The western blotting results revealed that metformin decreased the expression of EGFR, p-EGFR, AKT, and p-AKT, which could be reversed by EGF and SC79. Moreover, metformin activated AMPK and inactivated mTOR, and compound C and SC79 increased the levels of p-mTOR. Metformin can not only enhance the antitumour effect of cisplatin but also alleviate the toxic effects of cisplatin on the organs of xenograft model animals. In summary, the current study revealed that metformin inhibits the growth of SCLC by inducing autophagy and apoptosis via suppression of the EGFR/AKT/AMPK/mTOR pathway. Metformin might be a promising candidate drug for combination therapy of SCLC.
Keywords: Apoptosis; Autophagy; Cell cycle arrest; Metformin; SCLC.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: All animal experiments were approved by the Experimental Animal Ethics Committee of Hubei University of Medicine [2022-No.035].
Figures



References
-
- Govindan, R. et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol.24, 4539–4544. 10.1200/JCO.2005.04.4859 (2006). - PubMed
MeSH terms
Substances
Grants and funding
- C2022011/the Innovation Research Program for Graduates of Basic Medical College, Hubei University of Medicine
- JC2022014/the Innovation Research Program for Graduates of Basic Medical College, Hubei University of Medicine
- JC2023009/the Innovation Research Program for Graduates of Basic Medical College, Hubei University of Medicine
- S202210929020/the Hubei Provincial Training Program of Innovation and Entrepreneurship for Undergraduates
- 2018QDJZR11/the Cultivating Project for Young Scholars at Hubei University of Medicine
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

