Benefits of equilibrium between microbiota- and host-derived ligands of the aryl hydrocarbon receptor after stroke in aged male mice
- PMID: 39971928
- PMCID: PMC11839985
- DOI: 10.1038/s41467-025-57014-2
Benefits of equilibrium between microbiota- and host-derived ligands of the aryl hydrocarbon receptor after stroke in aged male mice
Abstract
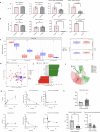
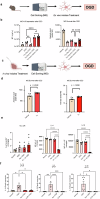
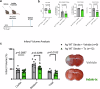
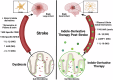
Recent studies have highlighted the crucial role of microglia (MG) and their interactions with the gut microbiome in post-stroke neuroinflammation. The activation of immunoregulatory pathways, including the aryl hydrocarbon receptor (AHR) pathway, is influenced by a dynamic balance of ligands derived from both the host and microbiota. This study aimed to investigate the association between stroke-induced dysbiosis and the resultant imbalance in AHR ligand sources (loss of microbiota-derived [indole-based] and increase of host-derived [kynurenine-based]) after stroke. Microbiota-derived AHR ligands decreased in human plasma and remained low for days following an ischemic stroke highlighting the translational significance. Transient-middle-cerebral-artery-occlusion was performed in aged wild-type and germ-free male mice. MG-AHR expression and activity increased in both in vivo and ex vivo stroke models. Germ-free mice showed altered neuroinflammation and antigen presentation while aged mice showed reduced infarct volume and neurological deficits following treatment with microbiota-derived AHR ligands after stroke. Restoring a balanced pool of host- and microbiota-derived AHR ligands may be beneficial after stroke and may represent a therapeutic target.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Update of
-
Restoring a balanced pool of host-derived and microbiota-derived ligands of the aryl hydrocarbon receptor is beneficial after stroke.Res Sq [Preprint]. 2023 Sep 13:rs.3.rs-3143015. doi: 10.21203/rs.3.rs-3143015/v1. Res Sq. 2023. Update in: Nat Commun. 2025 Feb 19;16(1):1767. doi: 10.1038/s41467-025-57014-2. PMID: 37790313 Free PMC article. Updated. Preprint.
References
-
- Virani, S. S. et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation141, e139–e596 (2020). - PubMed
-
- Benjamin, E. J. et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation137, e67–e492 (2018). - PubMed
-
- Powers, W. J. et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke50, e344–e418 (2019). - PubMed
-
- Rinaldo, L. et al. Racial and ethnic disparities in the utilization of thrombectomy for acute stroke. Stroke50, 2428–2432 (2019). - PubMed
MeSH terms
Substances
Grants and funding
- AG058463/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- 1R01AG070934-01/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 AG070934/AG/NIA NIH HHS/United States
- RF1 AG058463/AG/NIA NIH HHS/United States
- 1F31NS118984-01/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
LinkOut - more resources
Full Text Sources
Medical

