TREM2 promotes lung fibrosis via controlling alveolar macrophage survival and pro-fibrotic activity
- PMID: 39971937
- PMCID: PMC11840137
- DOI: 10.1038/s41467-025-57024-0
TREM2 promotes lung fibrosis via controlling alveolar macrophage survival and pro-fibrotic activity
Abstract
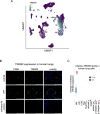
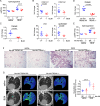
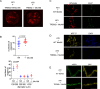
Lung macrophages play a pivotal role in pulmonary fibrosis, with monocyte-derived alveolar macrophages driving disease progression. However, the mechanisms regulating their pro-fibrotic behavior and survival remain unclear, and effective therapeutic strategies are lacking. Here we show that triggering receptors expressed on myeloid cells 2 are predominantly expressed on monocyte-derived alveolar macrophages in fibrotic mouse lungs and are significantly elevated in lung macrophages from patients with idiopathic pulmonary fibrosis. Deletion or knockdown of this receptor disrupts intracellular survival signaling, promotes macrophage apoptosis, and attenuates their pro-fibrotic phenotype. We further demonstrate that a lipid mediator and a high-avidity ligand of this receptor, encountered by macrophages in the alveolar milieu, enhance macrophage survival and activity. Ablation of TREM2 or blocking this receptor with soluble receptors or specific antibodies effectively alleviates lung fibrosis in male mice. These findings identify this receptor as a critical regulator of macrophage-mediated fibrosis and a promising therapeutic target for intervention.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

